Figures & data
Figure 1. Electrodes and treatment. Top: Electrode with two parallel rows of needles with 4 mm between the rows and a length of 20 mm (A) and electrode with a hexagonal array of needles with 7.9 mm between the needles with a length of 20 mm or 30 mm. (B). Bottom: Sixty-two-year-old woman with local recurrence of breast cancer on the left chest wall. In (C) the electrode is placed in the tumour, pulses are then delivered and the electrode is moved consecutively to deliver new pulses. In (D) the tumour after treatment is shown, needle marks after the electrode are seen as small red dots.
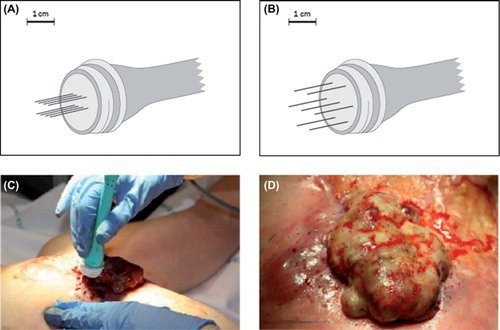
Table I. Patients’ characteristics at baseline.
Table II. Response evaluation.
Figure 2. Reduction in tumour volume after treatment with electrochemotherapy. Waterfall plot of tumour volume reduction on CT-scan. Twelve patients completed follow-up more than eight weeks, one patient was not evaluable by CT as tumour was indistinguishable from normal skin. Tumour volume of target lesions after one treatment with electrochemotherapy was compared with baseline tumour volume of target lesions and in four patients a reduction of more than 50% was observed.
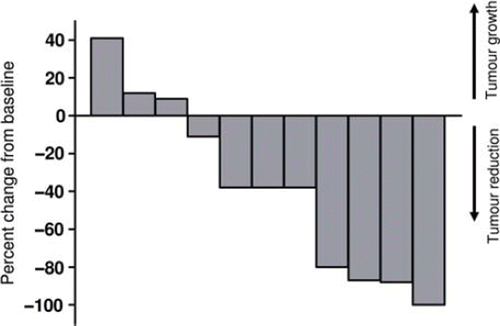
Figure 3. Patient treated with electrochemotherapy. Sixty-four-year-old woman with loco-regional recurrence of bilateral receptor negative, HER2 – negative breast cancer. Previous treatments over a period of five years included radiotherapy 48 Gy in 24 fractions on both sides and reirradiation with 30 Gy in 10 fractions on the left side, systemic therapy (cyclophosphamide, epirubicin, fluorouracil, docetaxel, gemcitabine, vinorelbine, and capecitabine). Despite all these treatments there was continuous progression of the cutaneous lesions. The column on the left shows image of lesions, CT-scan and PET/CT-scan before treatment scan, column on the right shows image of lesion, CT-scan and PET/CT-scan after two sessions with electrochemotherapy.
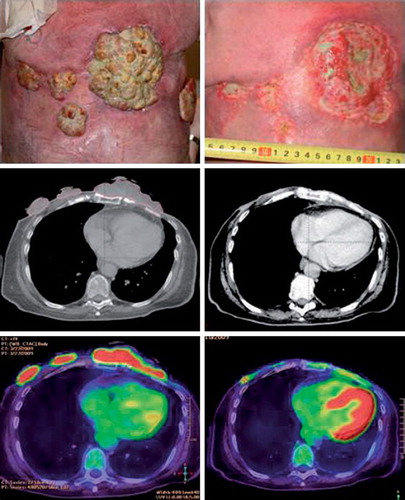
Table III. Toxicity in patients treated with electrochemotherapy.
Table IV. Patient reported symptoms.
