Figures & data
Table I. Odds ratios for the effects of clinical risk factors.
Table II. Summary of prevalence of clinical risk factors in the QUANTEC analysis.
Figure 1. Relationships between the mean dose to the lungs and the risk of radiation pneumonitis. Solid line: Dose-response reported in the QUANTEC study [Citation1]. Dashed line: Estimated dose-response for a patient currently smoking, with no risk factors (no pulmonary co-morbidities, no lower/middle tumor, below 63 years old, and no sequential chemotherapy). Dotted line: Estimated dose-response for patients with highest risk (pulmonary co-morbidities, middle or lower tumor location, no history of smoking, above 63 years of age, and sequential chemotherapy). Appropriate constraints on the mean lung dose in order to ensure a < 20% complication rate are marked on the primary axis.
![Figure 1. Relationships between the mean dose to the lungs and the risk of radiation pneumonitis. Solid line: Dose-response reported in the QUANTEC study [Citation1]. Dashed line: Estimated dose-response for a patient currently smoking, with no risk factors (no pulmonary co-morbidities, no lower/middle tumor, below 63 years old, and no sequential chemotherapy). Dotted line: Estimated dose-response for patients with highest risk (pulmonary co-morbidities, middle or lower tumor location, no history of smoking, above 63 years of age, and sequential chemotherapy). Appropriate constraints on the mean lung dose in order to ensure a < 20% complication rate are marked on the primary axis.](/cms/asset/717ef8fb-4233-423b-b55e-4263175543ad/ionc_a_820341_f0001_b.jpg)
Figure 2. Cumulative incidence functions for competing outcomes for patients in the validation cohort. Solid line: Radiation pneumonitis CTCAE v. 3.0 grade 3 or above. Dashed line: Local progression of lung cancer. Dotted line: Death from lung cancer. Dot-dashed line: Death from other (or unknown) cause.
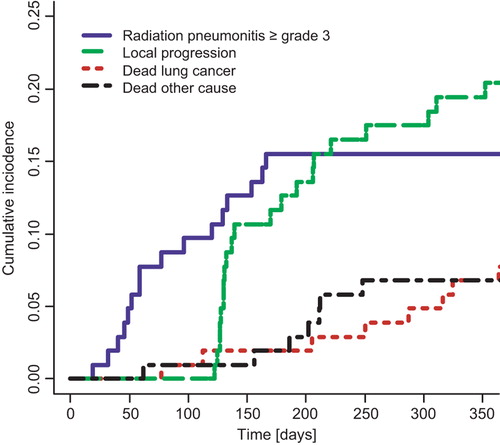
Table III. Patient characteristics in validation cohort.
Table IV. Crude incidence of radiation pneumonitis (as first event) in validation cohort.
Figure 3. Cumulative incidence of radiation pneumonitis for high/low risk patients in the validation cohort: Subdistribution cumulative incidence functions for radiation pneumonitis CTCEA v. 3.0 grade 3 or above. Solid line: Patients with predicted normal tissue toxicity (NTCP) below or equal to the group median (low-risk patients). Dashed line: Patients with NTCP above the group median (high-risk patients). Shaded areas: 95% confidence intervals. a) NTCP calculated using the dose-only relationship from the QUANTEC analysis [Citation1]. Group median: 17.0%. b) NTCP calculated using the individualized risk estimate, with the QUANTEC model adjusted for the effects of clinical risk factors. Group median: 13.4%.
![Figure 3. Cumulative incidence of radiation pneumonitis for high/low risk patients in the validation cohort: Subdistribution cumulative incidence functions for radiation pneumonitis CTCEA v. 3.0 grade 3 or above. Solid line: Patients with predicted normal tissue toxicity (NTCP) below or equal to the group median (low-risk patients). Dashed line: Patients with NTCP above the group median (high-risk patients). Shaded areas: 95% confidence intervals. a) NTCP calculated using the dose-only relationship from the QUANTEC analysis [Citation1]. Group median: 17.0%. b) NTCP calculated using the individualized risk estimate, with the QUANTEC model adjusted for the effects of clinical risk factors. Group median: 13.4%.](/cms/asset/0d805a88-7858-4893-8124-4d1e616a3591/ionc_a_820341_f0003_b.jpg)

