Figures & data
Figure 1. Phosphorylation of peptides by phosphoramidate as a function of time. The concentration of Ac-Val-Arg-Leu-Lys-His-Arg-Lys-Leu-Arg-pNA and Ac-Val-Arg-Leu-Lys-Ala-Arg-Lys-Leu-Arg-pNA was 0.5 mM and that of phosphoramidate 3.9 mM. The incubation was performed at room temperature and interrupted by centrifugation of 50 μL of the reaction mixture through two consecutive spin columns containing 210 μL DEAE-Sephacel equilibrated in 25 mM Tris/HCl, pH 8.0. The phosphate and peptide concentration in the final eluate was determined by malachite reagent and by measuring the absorbance at 320 nm, respectively. The values are given as means of duplicate analysis. (◊) Ac-Val-Arg-Leu-Lys-His-Arg-Lys-Leu-Arg-pNA and (▪) Ac-Val-Arg-Leu-Lys-Ala-Arg-Lys-Leu-Arg-pNA. Details are given in Material and methods.
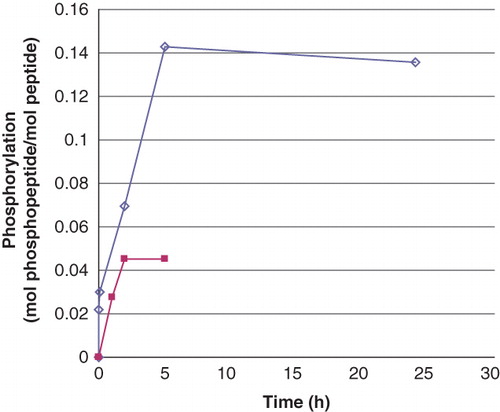
Figure 2. The PHPT1 activity as a function of enzyme concentration. The concentration of Ac-Val-Arg-Leu-Lys-His(P)-Arg-Lys-Leu-Arg-pNA was 24 μM in this experiment, and the enzyme was diluted 1/10 (▪) and 1/20 (◊), the former corresponding to 2.5 pmol per 50 μL incubation. The dephosphorylation was performed at 30°C and was interrupted by centrifugation of 50 μL of the reaction mixture through a spin column containing 210 μL DEAE-Sephacel equilibrated in 25 mM Tris/HCl, pH 8.0. The phosphate in the final eluate was determined by malachite reagent and peptide by absorbance at 320 nm. Details are given in Material and methods.
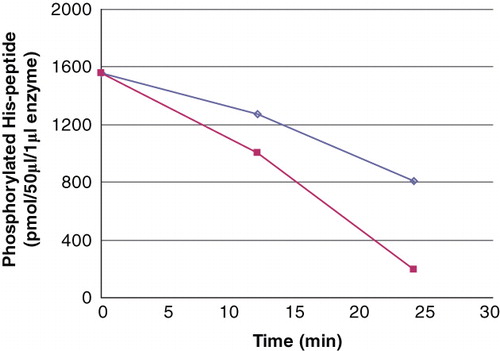
Figure 3. Activity of PHPT1 as a function of phosphopeptide concentration. After phosphorylation of 2 mM Ac-Val-Arg-Leu-Lys-His-Arg-Lys-Leu-Arg-pNA, phosphoramidate was removed by centrifugation through DEAE-Sephacel as described in Material and methods and diluted. The concentration of phosphopeptide was determined in each sample. Purified PHPT1 (1 pmol) was added to each concentration, and dephosphorylation was performed at 30°C in duplicate for 4, 8, and 12 min for each concentration. The reaction was interrupted by centrifugation of 50 μL through a spin column containing 210 μL DEAE-Sephacel equilibrated in 25 mM Tris/HCl, pH 8.0. The phosphate in the final eluate was determined by malachite reagent and peptide by absorbance at 320 nm. Details are given in Material and methods. The initial rate for each phosphopeptide concentration was plotted as a function of phosphopeptide concentration. The experiment was repeated three times with similar results.
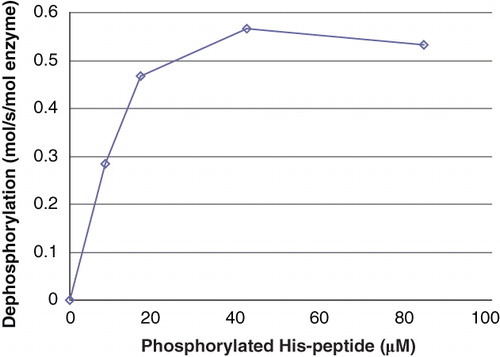
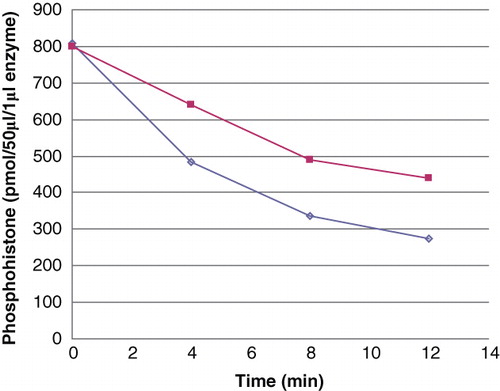
Figure 4. Dephosphorylation of purified and recombinant histone H4. Histone H4 was, after phosphorylation with phosphoramidate to 1.29 mol/mol, dephosphorylated with 2 pmoles PHPT1 for indicated times. The reaction was performed at 30°C and was interrupted by centrifugation of 50 μL of the reaction mixture through a spin column containing 210 μL DEAE-Sephacel equilibrated in 25 mM Tris/HCl, pH 8.5 at indicated times. Each time point was analysed in duplicate. The phosphate in the final eluate was determined by malachite reagent and histone by absorbance at 280 nm. Details are given in Material and methods.(◊) 40 μM purified phosphohistone H4, and (▪) 20 μM recombinant phosphohistone H4.