Figures & data
Figure 1. Production of VEGF, FGF, and PDGF in the tumor microenvironment. VEGF is produced by most cells in the tumor microenvironment, including endothelial cells and macrophages. It exerts its effects on endothelial cells; occasionally, tumor cells may express VEGF receptors and respond to VEGF. PDGF is produced by endothelial cells and serves to attract pericytes to embed the newly formed vessel; however, in the tumor, pericytes as a rule fail to wrap tightly around the endothelial cells. FGF is produced by tumors cells and may act directly on tumor vessels but not on endothelial cells in healthy tissues.
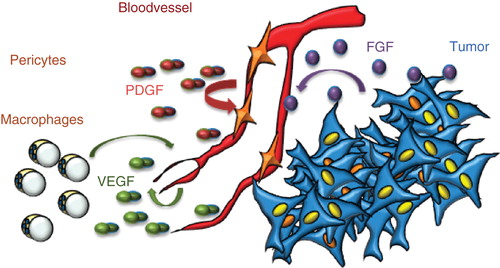
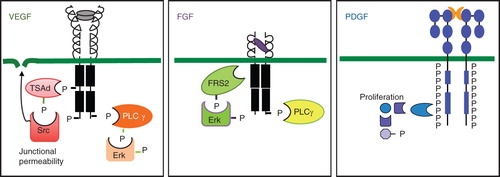
Figure 2. Properties of VEGF, FGF, and PDGF receptors and their signaling. Schematic outline of receptors is shown. All three VEGF receptors are organized into an extracellular domain with seven Ig-like folds and a short kinase insert. Tyrosine phosphorylation sites have been identified at some positions. Key features of VEGFR2 signaling are the binding of the adaptor molecule TSAd to the kinase insert phosphorylation site Y951 and regulation of Src activation followed by opening of adherens junctions. A C-terminal phosphorylation site at Y1175 allows binding of PLCγ and signaling in the Erk pathway. FGF receptors are composed of three Ig-like loops extracellularly, a very short kinase insert, and signaling via FRS2, which associates to the FGFR without involvement of tyrosine phosphorylation sites. PLCγ is an important downstream signal transducer in FGF biology. PDGF receptors have five extracellular Ig-like loops and a long kinase insert. The PDGF receptors become phosphorylated at very many tyrosine residues in response to ligand binding and induce formation of several long signaling chains. Erk = extracellular regulated kinase; FRS2 = FGF receptor substrate 2; P = phosphate; PLCγ = phospholipase Cγ; TSAd = T cell specific adaptor.