Figures & data
Figure 1. Disposition of patients used to evaluate the safety and efficacy of oral febuxostat for treatment of HLA-B*5801-negative gout.
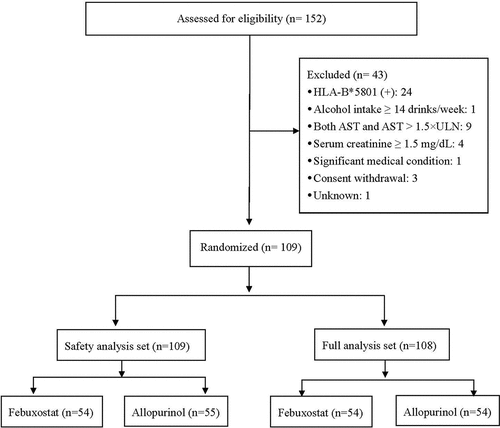
Table 1. Demographic and baseline characteristics of enrolled gout patients after the washout period (safety analysis set *).
Figure 2. Percentage change in serum urate level (mean ± sd) of gout patients before and at various times after initiation of treatment (day 1), full analysis set. * Significant difference (p < 0.05) based on a χ2 test.
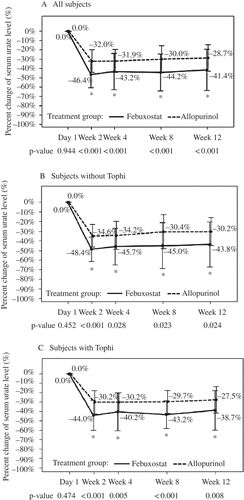
Table 2. Number (percentage) of enrolled gout patients with a serum urate level less than 6.0 mg/dL at different times after initiation of treatment (full analysis set *).
Table 3. Adverse events (AEs) of enrolled gout patients during the 12-week study period (safety analysis set).
Table 4. Changes in serum creatinine (safety analysis set).
