Open access
4,704
Views
31
CrossRef citations to date
0
Altmetric
Research Article
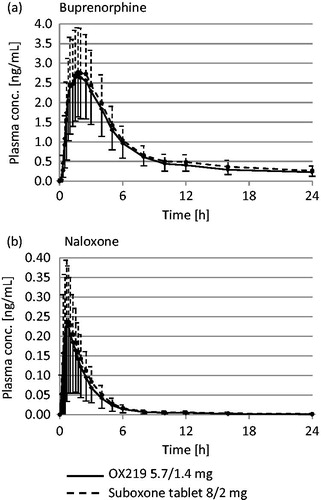
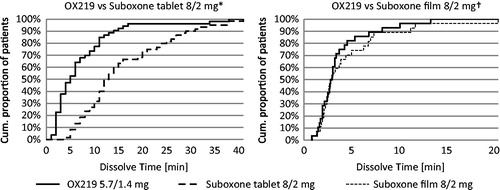
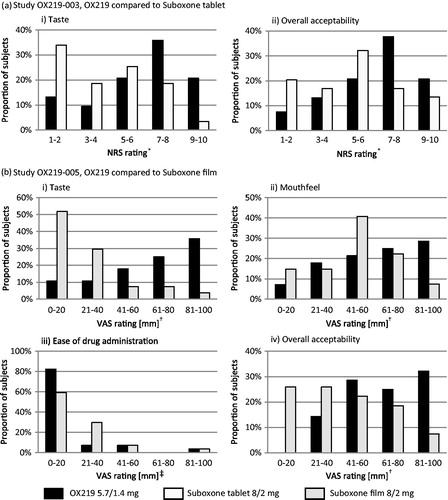
Pharmaceutical and pharmacokinetic characterization of a novel sublingual buprenorphine/naloxone tablet formulation in healthy volunteers
Andreas FischerOrexo AB, Uppsala, Sweden
, Martin JönssonOrexo AB, Uppsala, SwedenCorrespondence[email protected]
& Peter HjelmströmOrexo AB, Uppsala, Sweden
Pages 79-84
|
Received 20 Aug 2013, Accepted 16 Sep 2013, Published online: 07 Oct 2013
Related research
People also read lists articles that other readers of this article have read.
Recommended articles lists articles that we recommend and is powered by our AI driven recommendation engine.
Cited by lists all citing articles based on Crossref citations.
Articles with the Crossref icon will open in a new tab.