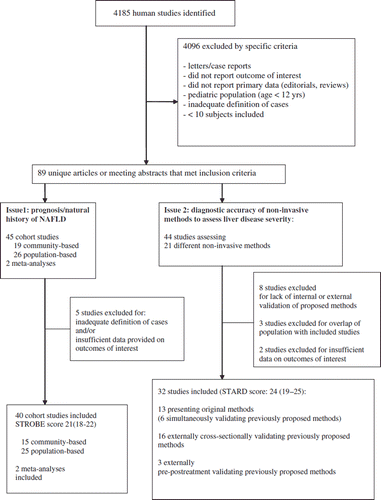
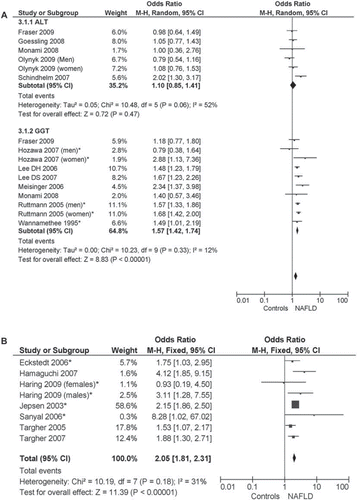
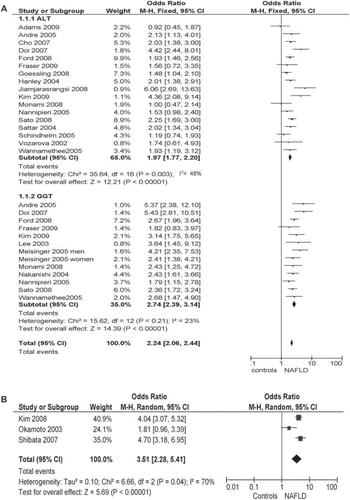
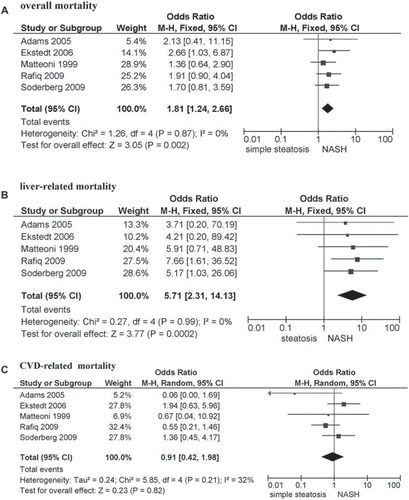
Figures & data
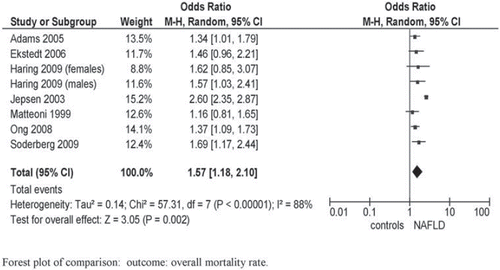
Table I. Prospective studies assessing the natural history of NAFLD, grouped according to definition of NAFLD (biochemical, radiological, or histological).
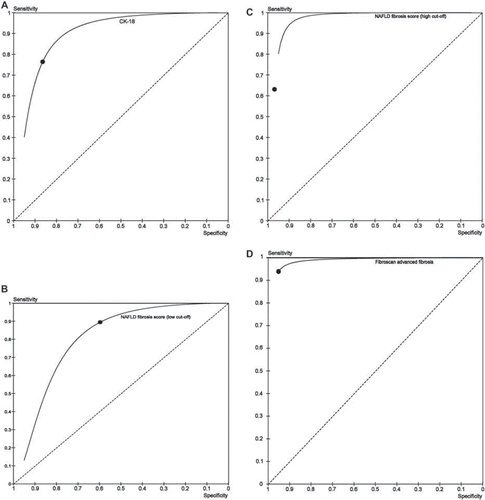
Table II. Panel A: Studies assessing biomarker panels validated for non-invasive assessment of the presence of NASH in patients with NAFLD. When > 1 study assessed the panel, summary estimates of diagnostic accuracy were calculated; when the panel was assessed in only 1 study, the AUROC is the summary estimate of the training and validation groups.
Table II. Panel B: Studies assessing biomarker panels validated for non-invasive assessment of fibrosis in patients with NAFLD. When > 1 study assessed the panel, summary estimates of diagnostic accuracy were calculated.
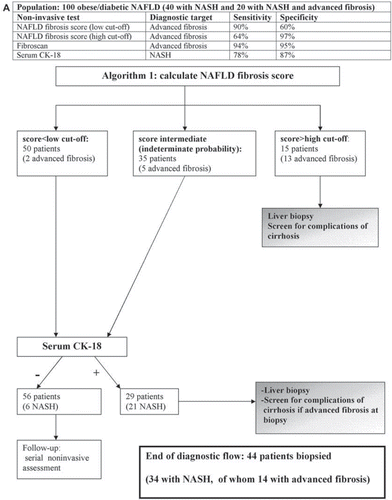
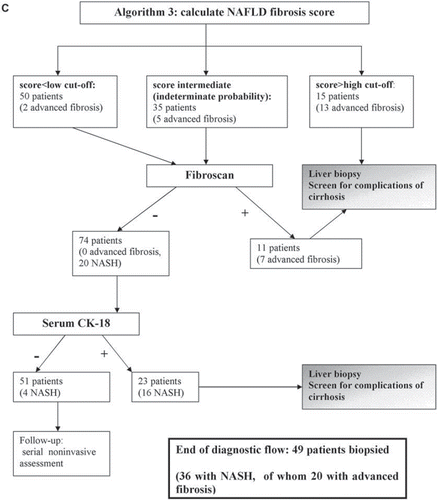
Table III. Suggested assessment of patients with NAFLD for general physicians.