Figures & data
Table I. Randomized clinical trials of dronedarone.
Figure 1. Benefits and risks of dronedarone to prevent the occurrence of atrial fibrillation (AF) or atrial flutter (AFL). The number of AF/AFL events avoided (black squares) and the number dronedarone discontinuations for a serious drug-related adverse event (gray circles) every 1,000 patients treated are plotted against the annual risk of AF recurrence with placebo in each trial. Solid lines indicate linear regressions of treatment effects. Extrapolation of regression lines (dotted lines) toward their intersection suggests that the antiarrhythmic benefits of dronedarone may not outweigh the risks associated with its use when the patients' annual risk of AF recurrence is smaller than 65%–70% (intersection of dotted lines). AEs = adverse events; ADONIS (Citation43) = American-Australian-African Trial with Dronedarone in Atrial Fibrillation or Flutter Patients for the Maintenance of Sinus Rhythm; EURIDIS (Citation43) = European Trial in Atrial Fibrillation or Flutter Patients Receiving Dronedarone for the Maintenance of Sinus Rhythm; DAFNE (Citation42) = Dronedarone Atrial Fibrillation Study After Electrical Cardioversion.
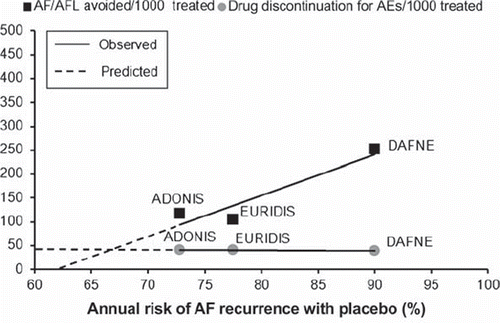
Figure 2. Benefits and risks of amiodarone to prevent the occurrence of atrial fibrillation (AF) or atrial flutter (AFL). The number of AF/AFL events avoided (black squares) and the number of amiodarone discontinuations for a serious drug-related adverse event (gray circles) every 1,000 patients treated are plotted against the annual risk of AF recurrence with placebo in each trial. Solid lines indicate linear regressions of treatment effects. Extrapolation of regression lines (dotted lines) does not show intersection, suggesting that the antiarrhythmic benefits of amiodarone outweigh the risks associated with its use across all patient populations. AEs = adverse events; GEFACA (Citation34) = Grupo de Estudio de Fibrilacion Auricular Con Amiodarona; SAFE-T (Citation15) = Sotalol Amiodarone Atrial Fibrillation Efficacy Trial; Kochiadakis et al. (Citation35); Channer et al. (Citation14).
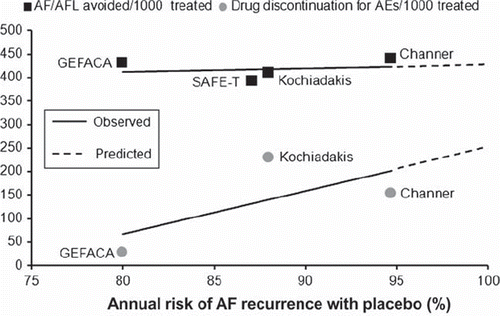
Figure 3. Effects of dronedarone on death or hospitalization for cardiovascular causes in different groups of patients. The number of death or hospitalization events (black squares) avoided (upper panel) or provoked (lower panel) every 1,000 patients treated is plotted against the annual risk of death or hospitalization with placebo in each trial. Solid line indicates linear regression of treatment effect and suggests an inverse relationship between dronedarone-associated benefits and base-line risk of patients, with harm for patients with an annual risk of death or hospitalization greater than 40%–45% (intersection of the regression line with the xaxis). The greatest benefit is observed in patients with low-to-moderate (25%–35%) annual risk of events. EURIDIS (Citation43) = European Trial in Atrial Fibrillation or Flutter Patients Receiving Dronedarone for the Maintenance of Sinus Rhythm; ADONIS (Citation43) = American-Australian-African Trial with Dronedarone in Atrial Fibrillation or Flutter Patients for the Maintenance of Sinus Rhythm; ATHENA (Citation47) = A Placebo-Controlled, Double-Blind, Parallel Arm Trial to Assess the Efficacy of Dronedarone 400 mg b.i.d.for the Prevention of Cardiovascular Hospitalization or Death from Any Cause in Patients with Atrial Fibrillation/Atrial Flutter; ANDROMEDA (Citation49) = Antiarrhythmic Trial with Dronedarone in Moderate to Severe CHF Evaluating Morbidity Decrease.
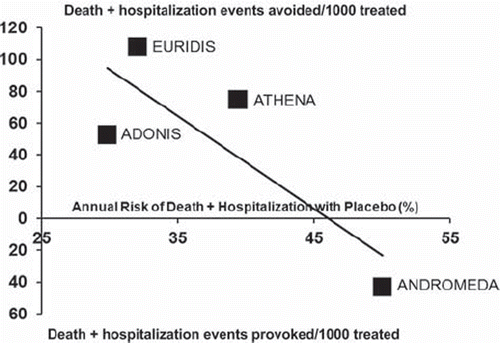
Figure 4. Ionic currents involved in the action potential of atrial myocytes. Atrial selective currents are highlighted in gray.
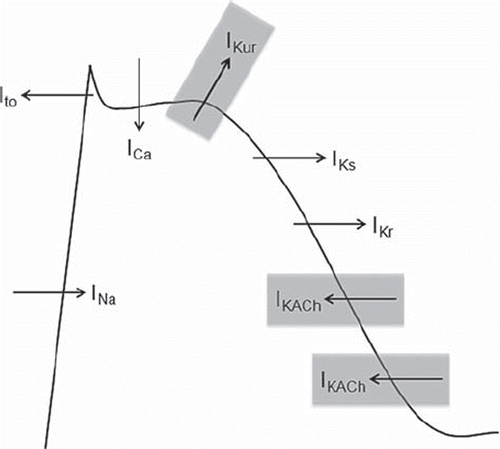
Table II. Summary of atrial selective agents.
Table III. Randomized clinical trials of vernakalant.
Figure 5. Indirect comparison of treatment effects of different intravenous antiarrhythmic agents for the acute conversion of atrial fibrillation (AF). SR = sinus rhythm.
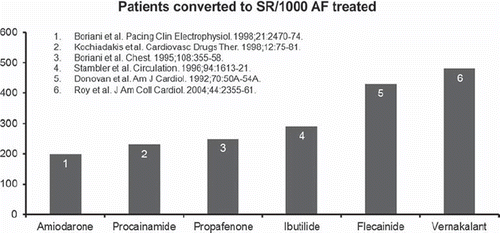
Figure 6. Putative molecular effects of upstream therapies for atrial fibrillation. RAA = renin-angiotensin-aldosterone.
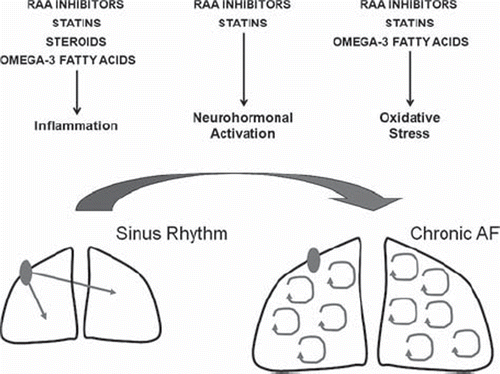
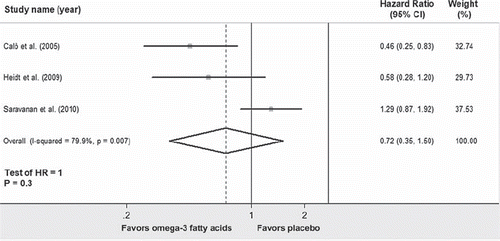
Figure 7. Forest plot showing the individual and pooled hazard ratios of postoperative atrial fibrillation comparing therapy with omega-3 fatty acids versus placebo. Square boxes denote hazard ratio; horizontal lines represent 95% confidence interval (CI).