Figures & data
Table I. Primary outcomes in selected randomized secondary prevention trials with statins.
Figure 1. Event rates in patient subgroups allocated simvastatin versus placebo in the Heart Protection Study (HPS) (Citation3). A: The rate of the primary end-point of all-cause mortality was significantly lower in patients allocated simvastatin (12.9%) versus placebo (14.7%) in the total study population in the HPS (12% relative risk reduction, 88% residual risk; P=0.003). B: The rate of the first major vascular event was significantly lower in patients receiving simvastatin (19.8%) than in those receiving placebo (25.2%) in the total study population (21% relative risk reduction, 79% residual risk; P<0.001), as well as in patients with prior myocardial infarction (MI) (23.5% versus 29.4%; 20% relative risk reduction, 80% residual risk; P<0.001), patients with cerebrovascular disease (18.7% versus 23.6%; 21% relative risk reduction, 79% residual risk; P<0.001), and patients with peripheral vascular disease (PVD) (24.7% versus 30.5%; 19% relative risk reduction, 81% residual risk; P<0.001). Green arrows indicate relative risk reduction between treatment arms. Red arrows indicate residual risk for recurrent events.
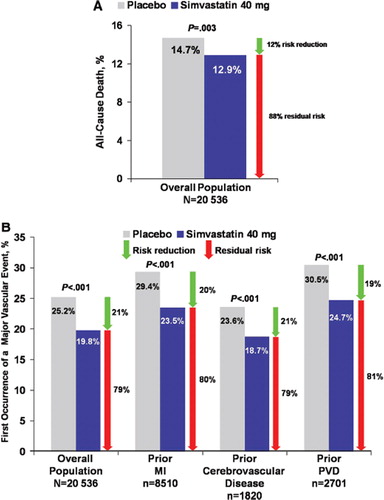
Figure 2. Primary end-point rate in the CHARISMA trial (Citation83,Citation84). No significant difference was obtained in the primary end-point rate (composite of myocardial infarction, stroke, or death from cardiovascular causes) between placebo+aspirin versus clopidogrel+aspirin groups in the overall study population and in the primary prevention cohort (Citation83). Significant reductions in favor of clopidogrel+aspirin were evident in the secondary prevention cohort (Citation83) and in the CAPRIE-like population (Citation84). Green arrows indicate relative risk reduction between treatment arms. Red arrows indicate residual risk for recurrent events.
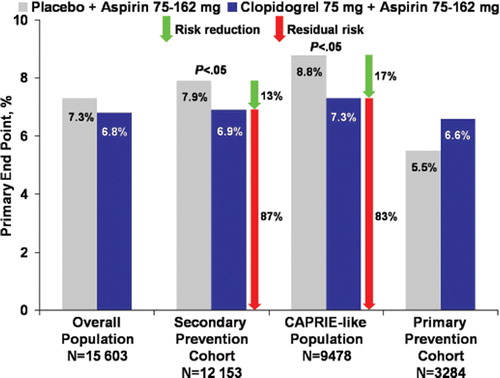
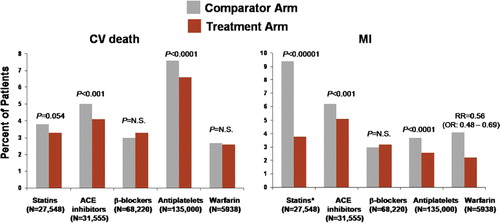
Figure 3. Residual atherothrombotic risk: rates of cardiovascular (CV) death and myocardial infarction (MI) persisting with active treatments from meta-analyses of trials of secondary prevention with statins (Citation52), angiotensin-converting enzyme (ACE) inhibitors (Citation60), β-blockers (Citation71), antiplatelets (Citation2), and warfarin (Citation80). Treatment and comparator arms consisted of the following: high-dose versus standard-dose statins; ACE inhibitor versus placebo; β-blocker versus placebo or other antihypertensive agents; antiplatelet therapy versus control; and warfarin+aspirin versus aspirin. *Event rate is shown for coronary death/MI for statin meta-analysis (individual rates for MI not available). OR = odds ratio.