Figures & data
Table I. Questionnaire.
Table II. Baseline characteristics.
Figure 1. Proportion (%) of controlled subjects and responders under nebivolol in each treatment category (monotherapy, add-on, switch) at visits 2 and 3: proportion of JNC-VII goal achievers (dark column), proportion of subjects with SBP/DBP decreased by more than 10/5 mmHg (white column), and proportion of responders defined by SBP/DBP decreased by more than 10/5 mmHg irrespective of JNC-VII target thresholds attainment and/or those who were considered as BP controlled (grey column)
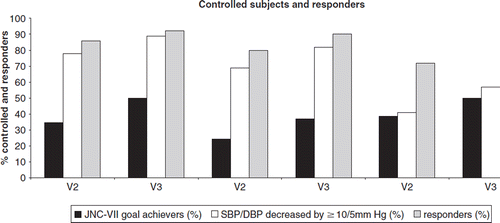
Table III. Quantitative changes in Quality of life items.
Table IV. Qualitative changes in Quality of life (QoL) items.