Figures & data
FIGURE 1. The Lacrimal Functional Unit. The LFU unifies the complex reflex network connecting the sensory tissues and secretory glands that provide homeostasis on the ocular surface, and is composed of the ocular surface tissues (cornea, corneal limbus, conjunctiva, conjunctival blood vessels, and eyelids), the tear secreting machinery (main and accessory lacrimal glands, meibomian glands, conjunctival goblet, and epithelial cells), and their neural connections. The LFU is tightly controlled by neural input from the ocular surface tissues. Subconscious stimulation of the corneal nerve endings triggers afferent impulses through the ophthalmic branch of the trigeminal nerve (V), which integrate in the central nervous system and the paraspinal sympathetic tract, in turn generating efferent secretomotor impulses that stimulate secretion of the healthy tear film. Any one of several sensory stimuli, e.g., pain, microbial/environmental insult, and emotion can stimulate the tear secreting reflex. Illustration from Beuerman et al. The Lacrimal Functional Unit in Dry Eye and Ocular Surface Disorders (eds. Pflugfelder SC, Beuerman RW, and Stern ME) (Marchel Dekker, Inc., New York, 2004) 11–39.
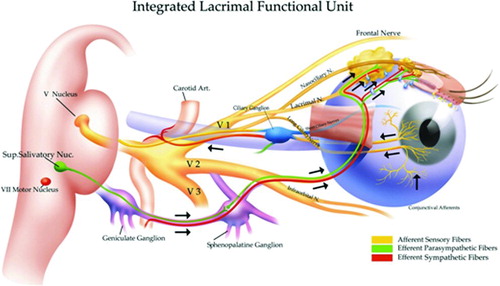
FIGURE 2. Inflammatory circle of chronic dry eye. Stress to the ocular surface triggers the initial events leading to localized autoimmunity. Acute response cytokines, such as TNF-α, IL-1α, IL-1β, and IL-6 further enhance proinflammatory cytokine/chemokine production, adhesion molecule expression required for innate cell infiltration, and also activate resident antigen presenting cells (APCs). Mature APCs home to the regional lymph nodes to activate Th1 and Th17 cells. Autoreactive T cells traffic to the ocular surface tissues where they potentiate the chronic autoimmune response and cause pathology. For example, IFN-γ alters mucins on corneal epithelial cells and is linked to epithelial cell apoptosis, goblet cell loss, and squamous metaplasia. IL-17 increases MMP3/9 expression and induces corneal epithelial barrier dysfunction. In addition, recent data suggest that autoantibodies bind to antigens expressed in the LFU to cause complement-dependent tissue destruction.
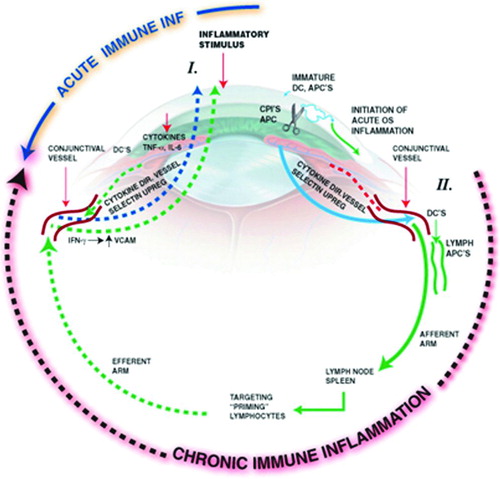
TABLE 1. Tear components supporting corneal health.
TABLE 2. Stress-specific expression of proinflammatory mediators in corneal and conjunctival epithelium.
FIGURE 3. γδ T cells and upregulation of IL-17 on the ocular surface early during the immunopathogenesis of desiccating stress (DS)-induced dry eye disease. (A) Immunohistochemistry on sagittal sections of whole eyes showed positive staining for γδ T cells using purified hamster anti-mouse γδ T cell receptor (8.75 μg/ml; BD Pharmingen). Images captured at 40×. (B) Reproduced from Zhang et al. [Citation106] mRNA levels in NK/NKT positive (+) and NK/NKT negative (–) ocular surface cells isolated from nonstressed (NS) spleen and ocular surface (OS) and at different time points after DS. Unfractionated spleen was used as a calibrator. *indicates p <0.05, ***indicates p < 0.001 comparison versus NS control NK/NKT+. ∧ indicates p < 0.05, ∧∧∧ indicates p < 0.001 comparison versus NS control NK/NKT. (C) Tear levels of IL-17. Relative levels of IL-17 in the tears of dry eye using Luminex analysis as previously described [Citation71]. (B and C) DS; DS1 = DS for 1 day, DS3 = DS for 3 days, DS5 = DS for 5 days, DS10 = DS for 10 days.
![FIGURE 3. γδ T cells and upregulation of IL-17 on the ocular surface early during the immunopathogenesis of desiccating stress (DS)-induced dry eye disease. (A) Immunohistochemistry on sagittal sections of whole eyes showed positive staining for γδ T cells using purified hamster anti-mouse γδ T cell receptor (8.75 μg/ml; BD Pharmingen). Images captured at 40×. (B) Reproduced from Zhang et al. [Citation106] mRNA levels in NK/NKT positive (+) and NK/NKT negative (–) ocular surface cells isolated from nonstressed (NS) spleen and ocular surface (OS) and at different time points after DS. Unfractionated spleen was used as a calibrator. *indicates p <0.05, ***indicates p < 0.001 comparison versus NS control NK/NKT+. ∧ indicates p < 0.05, ∧∧∧ indicates p < 0.001 comparison versus NS control NK/NKT. (C) Tear levels of IL-17. Relative levels of IL-17 in the tears of dry eye using Luminex analysis as previously described [Citation71]. (B and C) DS; DS1 = DS for 1 day, DS3 = DS for 3 days, DS5 = DS for 5 days, DS10 = DS for 10 days.](/cms/asset/f544acd1-3acb-481f-81f7-3bd3ebebb46f/iiri_a_748052_f0003_b.jpg)
FIGURE 4. Desiccating stress-induced model of dry eye. Female C57BL/6 mice (6–8 weeks old) exposed to desiccating stress (DS: subcutaneous scopolamine (1 mg/0.2 ml) three times per day, humidity <40%, and sustained airflow) display similar clinical and histopathological features to patients with dry eye disease. Moreover, CD4+ T cells isolated from the cervical lymph nodes and spleen of dry eye mice are sufficient to cause disease in T-cell-deficient nude-recipient mice. Desiccating stress-induced model of dry eye was originally described in Dursun et al. 2002. IOVS. 43[Citation3]:632–638 and Niederkorn et al. 2006. J. Immunol. 176: 3950–3957 (70;131).
![FIGURE 4. Desiccating stress-induced model of dry eye. Female C57BL/6 mice (6–8 weeks old) exposed to desiccating stress (DS: subcutaneous scopolamine (1 mg/0.2 ml) three times per day, humidity <40%, and sustained airflow) display similar clinical and histopathological features to patients with dry eye disease. Moreover, CD4+ T cells isolated from the cervical lymph nodes and spleen of dry eye mice are sufficient to cause disease in T-cell-deficient nude-recipient mice. Desiccating stress-induced model of dry eye was originally described in Dursun et al. 2002. IOVS. 43[Citation3]:632–638 and Niederkorn et al. 2006. J. Immunol. 176: 3950–3957 (70;131).](/cms/asset/ad5f55e1-1ff9-4356-b299-fae9a63c255e/iiri_a_748052_f0004_b.jpg)
FIGURE 5. Antigen-presenting cell depletion in mice exposed to desiccating stress attenuating the generation of autoreactive CD4+ T cells and blocking the ability to adoptively transfer T-cell-mediated disease to nude-recipient mice. CD4+ T cells were isolated from the CLN of clodronate- or PBS-treated mice exposed to DS and adoptively transferred to nude-recipient mice to determine if the absence of a full repertoire of APCs within the ocular surface tissues inhibits generation of ocular surface-specific autoreactive CD4+ T cells. (A) H&E staining showed a (B) significant decrease in overall inflammatory cell infiltration within the ocular surface tissues of nude recipients of CD4+ T cells from clodronate-treated donor mice compared to PBS controls (3 days postadoptive transfer). Furthermore, (C) IHC confirmed that CD4+ T cells isolated from clodronate-treated donor mice did not readily accumulate within the conjunctiva as there was only trace CD4+ staining, which accounted for a (D) significant decrease in CD4+ T cells and (E, F) protection from the loss of PAS-positive goblet cells. (A) H&E staining; (B) overall inflammatory score ± SEM (scale 0–3); (C) CD4+ T cell staining in the conjunctiva; (D) is average conjunctival (conj.) CD4+ T-cell counts ± SEM; (E) PAS+ goblet cell staining in the conjunctiva; (F) is average conjunctival goblet cell counts ± SEM. The data are representative of three independent experiments, with an n = 5–6 mice/group. Statistically significant values (*p ≤ 0.05, **p ≤ 0.01) are indicated relative to nude recipients of CD4+ T cells from PBS liposome-treated mice. Reproduced from Schaumburg et al. 2011. J Immunol. 187[Citation7]:3653–3662 [Citation59].
![FIGURE 5. Antigen-presenting cell depletion in mice exposed to desiccating stress attenuating the generation of autoreactive CD4+ T cells and blocking the ability to adoptively transfer T-cell-mediated disease to nude-recipient mice. CD4+ T cells were isolated from the CLN of clodronate- or PBS-treated mice exposed to DS and adoptively transferred to nude-recipient mice to determine if the absence of a full repertoire of APCs within the ocular surface tissues inhibits generation of ocular surface-specific autoreactive CD4+ T cells. (A) H&E staining showed a (B) significant decrease in overall inflammatory cell infiltration within the ocular surface tissues of nude recipients of CD4+ T cells from clodronate-treated donor mice compared to PBS controls (3 days postadoptive transfer). Furthermore, (C) IHC confirmed that CD4+ T cells isolated from clodronate-treated donor mice did not readily accumulate within the conjunctiva as there was only trace CD4+ staining, which accounted for a (D) significant decrease in CD4+ T cells and (E, F) protection from the loss of PAS-positive goblet cells. (A) H&E staining; (B) overall inflammatory score ± SEM (scale 0–3); (C) CD4+ T cell staining in the conjunctiva; (D) is average conjunctival (conj.) CD4+ T-cell counts ± SEM; (E) PAS+ goblet cell staining in the conjunctiva; (F) is average conjunctival goblet cell counts ± SEM. The data are representative of three independent experiments, with an n = 5–6 mice/group. Statistically significant values (*p ≤ 0.05, **p ≤ 0.01) are indicated relative to nude recipients of CD4+ T cells from PBS liposome-treated mice. Reproduced from Schaumburg et al. 2011. J Immunol. 187[Citation7]:3653–3662 [Citation59].](/cms/asset/f7d1fac7-0bdd-411e-ad90-592139654620/iiri_a_748052_f0005_b.jpg)
FIGURE 6. Working Hypothesis: the contribution of autoreactive B cells during dry eye. 1) Autoreactive B cells recognize putative dry eye autoantigens through B-cell receptor interactions (e.g., M3R, Klk13), [Citation2] intenalize the antigen, and [Citation3] subsequently present the antigen to [Citation4] Th2 cells: cytokines derived from activated Th2 cells promote B-cell differentiation to [Citation5] plasma cells, which [Citation6] produce autoantibodies (e.g., anti-M3R, anti-Klk13) that [Citation7] cause complement-dependent tissue damage.
![FIGURE 6. Working Hypothesis: the contribution of autoreactive B cells during dry eye. 1) Autoreactive B cells recognize putative dry eye autoantigens through B-cell receptor interactions (e.g., M3R, Klk13), [Citation2] intenalize the antigen, and [Citation3] subsequently present the antigen to [Citation4] Th2 cells: cytokines derived from activated Th2 cells promote B-cell differentiation to [Citation5] plasma cells, which [Citation6] produce autoantibodies (e.g., anti-M3R, anti-Klk13) that [Citation7] cause complement-dependent tissue damage.](/cms/asset/a140271c-d15c-4262-ab16-ebd60aa4f0be/iiri_a_748052_f0006_b.jpg)
TABLE 3. Dry eye disease severity and treatment strategy.