Figures & data
FIGURE 1. Schematic representation of the family of group V type II CTLRs known as the Dectin-1 cluster. A: The activatory receptors Dectin-1, CLEC-2 and CLEC-9A containing ITAM-like and, with the exception of CLEC-9A, tri-acidic motifs important for downstream signaling. B: The inhibitory receptors MICL and MAH containing canonical ITIM motifs. C: Receptors containing novel motifs including C-type lectin-like scavenger receptor LOX-1, containing a DDL motif and CLEC-1 containing an uncharacterised tyrosine-based motif as well as a tri-acidic motif.
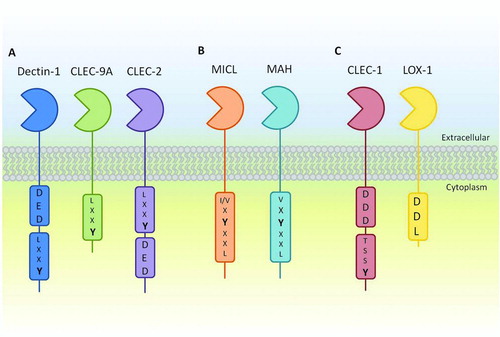
FIGURE 2. Signaling network of Dectin-1. Multiple pathways downstream of Dectin-1 rely on Syk. This can lead to PLCγ2/Ca2+ signaling leading to PKCδ activation that controls calcineurin/NFAT and ROS/NLRP3 inflammasome, and the phosphorylation of CARD9-Bcl10-Malt1. The CARD9 complex is able to activate all NF-κB subunits including c-Rel by removing its inhibitor, IκB, through activation of the IKK complex. Syk can also activate IKK complex through the non-canonical NIK pathway, leading to the activation of NF-κB subunits RelB and p52. This pathway can be inhibited the Raf-1-mediated pathway involving p65. Phosphorylated and acetylated p65 can interact with RelB, sequestering it away from an active NF-κB complex. Resultant activation of NFAT and NF-κB results in modification of gene transcription including cytokines and chemokines. Dashed arrows represent a pathway that has yet to be fully defined.
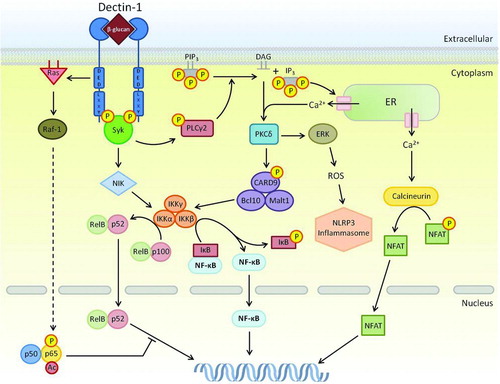
FIGURE 3. ITAM-like-bearing CTLRs within the Dectin-1 cluster. As well as Dectin-1, there are two additional receptors that contain ITAM-like motifs. These receptors share Syk as a primary downstream signaling kinase; however, the lack of a tri-acidic in CLEC-9A and the presence of an isoleucine prevent the endocytic and activation functions of this receptor. CLEC-2 utilises its tri-acidic motif to complement activation through Syk resulting in adhesion and clot formation in platelets. Dashed arrows represent a pathway that has yet to be fully defined.
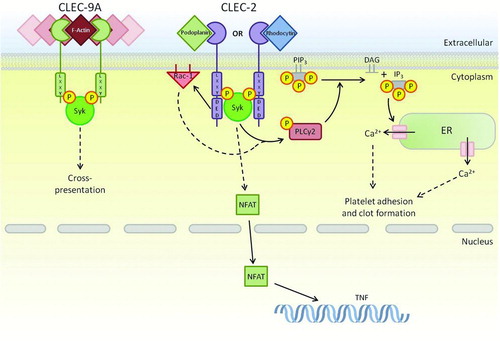
FIGURE 4. ITIM-bearing CTLRs within the Dectin-1 cluster. The ITIM motif within MICL and MAH is known to interact with SHP-1 and SHP-2; however, pathways downstream of these phosphatases are only partially known. MICL is known to inhibit cytokine transcription instigated by other receptors as a method of control. On the other hand, there is evidence to suggest that MICL induces activatory pathways through MAPK, which is somewhat contradictory to the known functions of its motif. Downstream signaling of MAH is largely unknown. Dashed arrows represent a pathway that has yet to be fully defined.
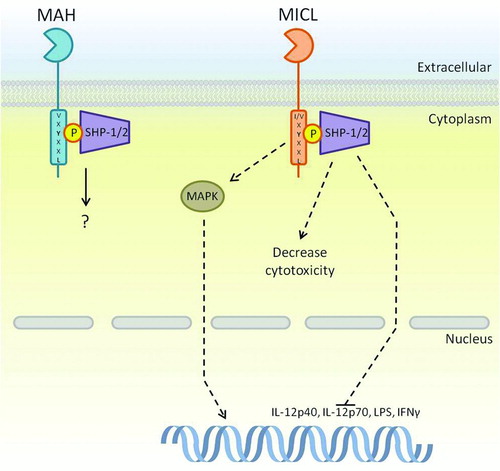
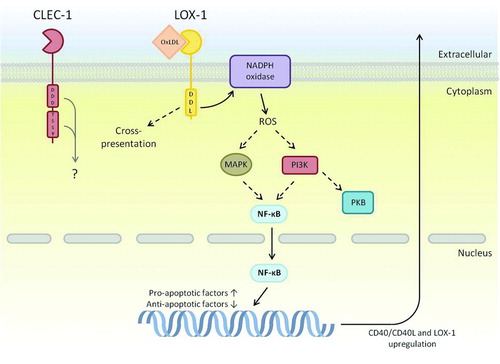
FIGURE 5. CTLRs within the Dectin-1 cluster that contain novel signaling motifs. Many components downstream of LOX-1s novel DDL motif have been determined, although exact mechanisms are not entirely identified. Interestingly, LOX-1 has the ability to up-regulate transcription of other receptors including itself; a mechanism that has been shown to aid in plaque formation during atherosclerosis. Downstream signaling of CLEC-1 is largely unknown; however, it does contain a tri-acidic motif that may have signaling potential due to similar signaling sequences in Dectin-1 and CLEC-2. Dashed arrows represent a pathway that has yet to be fully defined, and the grey arrow represents potential pathways based on other related receptor functions.