Figures & data
Table 1. Clinical data of Anderson-Fabry disease patients
Figure 1. Comparison of 2D electrophoreograms of urine samples collected from patients with Fabry disease (A, B) and healthy controls (C, D). The urine samples were analyzed immediately after their collection and short-time centrifugation, using 7 cm polyacrylamide strips, pH 3–10 L, and SDS-PAGE. BenchMarkTM Protein Ladder (Invitrogen, Glasgow, UK) was used as a molecular mass marker.
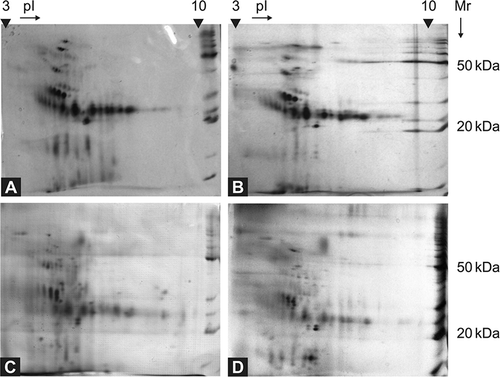
Table 2. Changes of selected proteins observed using Phoretix 2D expression software version 2005
Figure 2. Changes in the protein amount in the samples collected from 20 patients with Fabry disease compared with those in healthy controls. *p < 0.05 versus healthy controls.
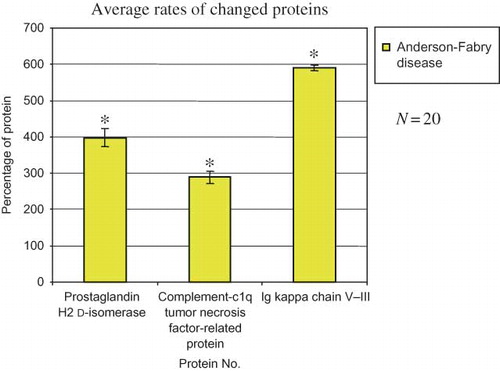
Figure 3. A representative 2D gel image of the Fabry disease proteome, depicting all identified proteins. All urine samples were analyzed immediately after their collection using 7 cm polyacrylamide strips, pH 3–10 L, and SDS-PAGE.
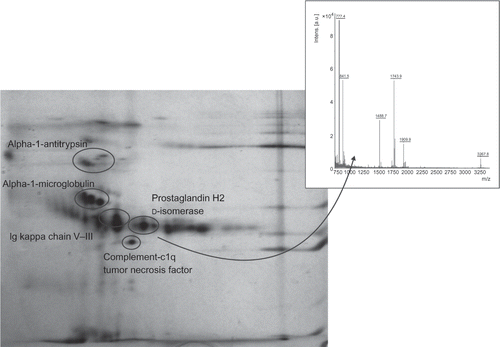
Table 3. Comparison of experimental and theoretical molecular size and isoelectric point of identified proteins by MALDI-TOF MS