Figures & data
Figure 1. Our center's protocol for ABO-incompatible kidney transplantation (ABO IKT). We used RTX at 30 days before transplantation and started plasmapheresis and intravenous immunoglobulin (PP/IVIG) at 15–20 days before KT. We started immunosuppressants composed of tacrolimus, prednisone, and mycophenolate mofetil at 7 days before KT. CD19- and CD20-cell counts were measured before and at 14 days after RTX infusion. Isoagglutinin test for anti-ABO antibody was checked daily from the start of PP/IVIG to second week from KT.
Note: RTX, rituximab; MMF, mycophenolate mofetil; PP/IVIG, plasmapheresis/intravenous immunoglobulin.
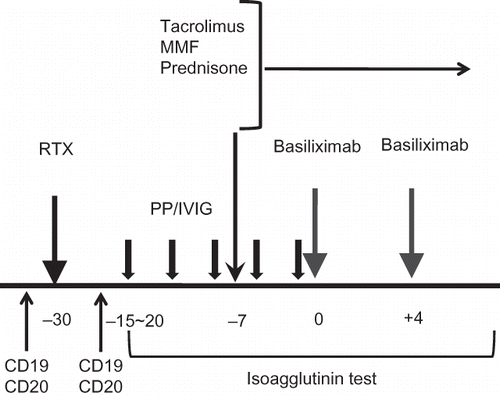
Figure 2. (A) Modified protocol for patients with extremely high baseline titer (1:1024). In addition to routine protocol, we used additional dose of RTX (375 mg/m2) at 7 days before KT in high-titer 1 and 4. (B) Another modified protocol for patients with extremely high baseline titer (1:1024). In addition to the routine protocol, we used two times of bortezomib at 14 days and 10 days before KT in high-titer 8. (C) Modified protocol for highly sensitized and ABO-incompatible transplantation (ABO IKT) in highly sensitized patients (low-titer 6). In addition to routine protocol for ABO IKT in our center, high-dose intravenous immunoglobulin (2 g/kg) at 28 days before KT and additional dose of RTX at 7 days before KT were used. For the monitoring of donor-specific HLA antibody, Luminex method was used at every week from 3 weeks before KT until 1 week after KT. MFI of the donor-specific anti-HLA antibody gradually decreased from >10,000 to less than 2000 by preparation protocol.
Note: RTX, rituximab; PP/IVIG, plasmapheresis/intravenous immunoglobulin; ATG, anti-thymocyte globulin. MFI, median fluorescent intensity.
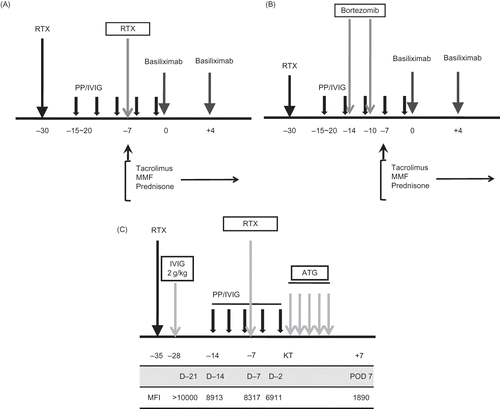
Table 1. Comparison of the baseline characteristics between the high-titer and low-titer groups
Table 2. Comparison of the preparation intensity between the high-titer and low-titer groups
Figure 3. Change of CD19-positive cell counts (A) CD20-positive cell counts (B) before and after RTX infusion. In all cases, CD19-/CD20-positive cell counts were successfully depleted to less than 1%.
Note: RTX, rituximab.
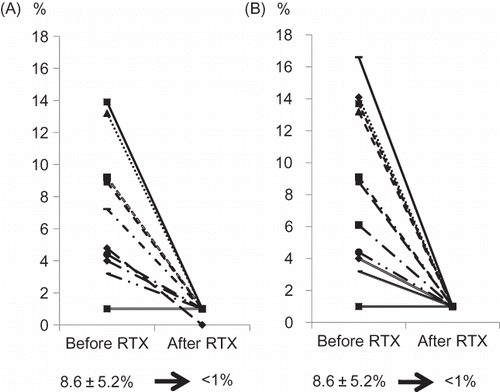
Figure 4. Comparison of the change of Scr level between the high-titer and low-titer groups. No significant difference was found between two groups until 6 months after KT in Scr level.
Note: Scr, serum creatinine; KT, kidney transplantation.
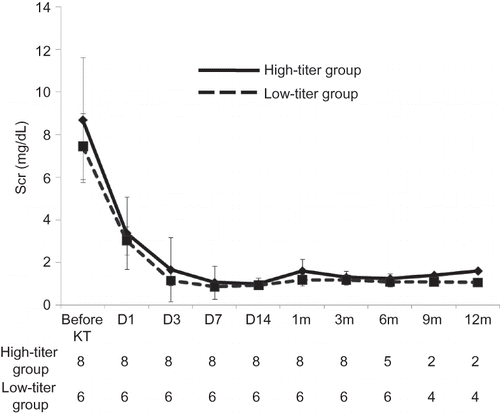
Figure 5. Comparison of the change of anti-ABO antibody titer (IgG) between the high-titer and low-titer groups. Represented value is the median level of antibody titer at each period. At baseline, antibody titer was significantly higher in the high-titer group, but the difference significantly decreased at transplantation. Afterward no significant difference was found between two groups until 6 months after KT. We only present IgG level because IgG titer was higher than IgM during entire period.
Note: KT, kidney transplantation.
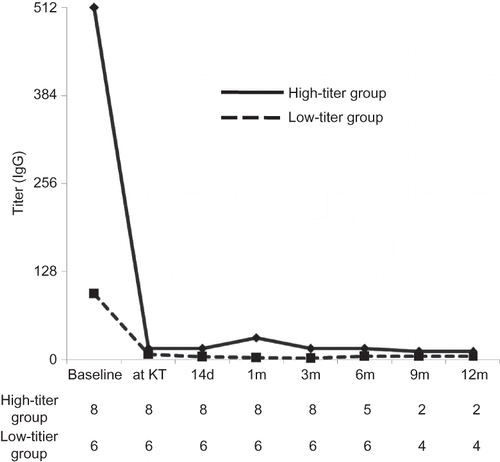
Table 3. Comparison of the results of protocol biopsy between the high-titer and low-titer groups
Table 4. Comparison of complications during follow-up period between the high-titer and low-titer groups