Figures & data
Figure 1 Scanning electron microscopy (SEM) photos of a polyethylene glycol coated collagen pad (PCC, Hemopatch) and a fibrin and thrombin coated collagen pad (FTC, TachoSil). (A, B) Cross sectional images show the open porous structure of PCC compared to (C, D) the closed-cell honeycomb like matrix of FTC. (A) The NHS-PEG coating on PCC and (C) the human protein coating on FTC are on the right surface (arrows). (A, C) Lower magnification (B, D) higher magnification.
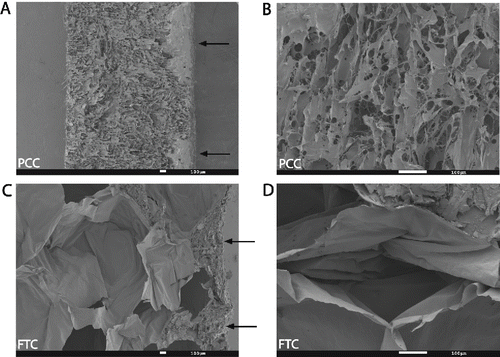
Figure 2 Clopidogrel and acetylsalicylic acid (ASA) statistically significantly inhibited platelet function (p < .001). Platelet aggregation in PRP was assessed using (A) a collagen- and (B) adenosine diphosphate (ADP)-triggered platelet aggregation assay. Results expressed as the mean of duplicate measurements for individual animals before (white bars) and after (black bars) a 5-day administration of antiplatelet therapy are shown.
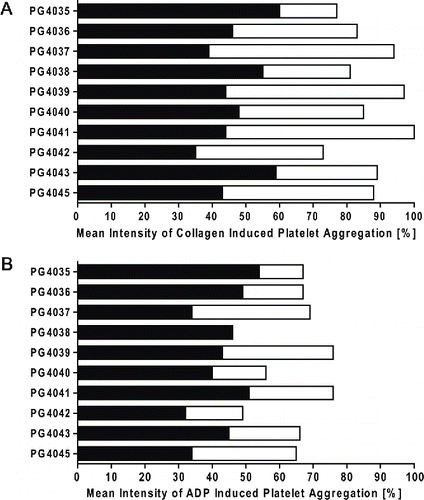
Figure 3 Superior hemostatic properties of PCC, a polyethylene glycol coated collagen pad relative to FTC, a fibrinogen-thrombin coated collagen pad. (A) Kaplan-Meier plots for interval-censored time to hemostasis in animals treated with PCC without antiplatelet therapy; (B) Kaplan–Meier plots for interval-censored time to hemostasis (PCC, solid line; FTC, dashed line) per animal on dual antiplatelet therapy; grey boxes represent time interval during which hemostasis was achieved (3–4 lesions per group per animal).
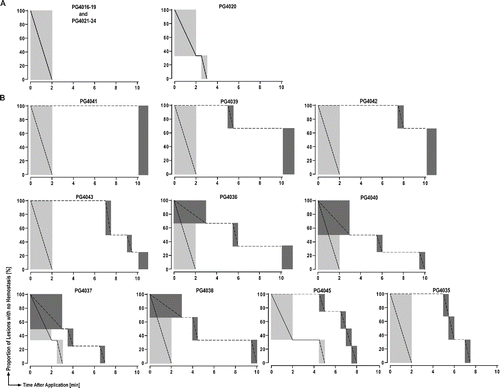
TABLE 1 Hemostasis properties of PCC versus FTC
Figure 4 (A) Statistical model-estimated probability of hemostasis over time with PCC, a polyethylene glycol coated collagen pad, with (solid line) and without (dashed line) antiplatelet therapy calculated based on a median bleeding rate of 150 ml. (B) Statistical model-estimated probability of hemostasis over time with PCC (solid line) relative to FTC, a fibrinogen-thrombin coated collagen pad (dashed line) calculated based on the median bleeding rate of 127 ml/min in animals on dual antiplatelet therapy. Time to hemostasis was 12 times shorter (95% CI: 1 to 102) with PCC than with FTC.
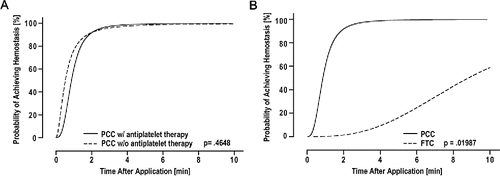
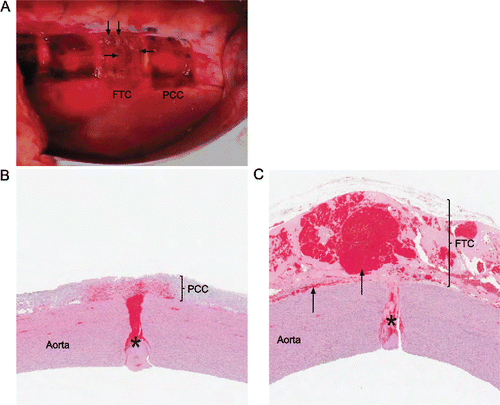
Figure 5 Sutureless aortotomy repair. (A) Hemostatic agents 10 min after application in situ during surgery (left is cranial and top is dorsal; cranial pad is FTC and caudal pad is PCC); Hematoma formation over incision (arrows). (B, C) Representative images of hematoxylin and eosin-stained aortic cross sections; x10. (B) Clot formation (asterisk), flush adherence to aortic tissue, and tight sealing with PCC. (C) Clot formation (asterisk), and increased sub- and intrapatch blood accumulation (arrows) with FTC.