Figures & data
FIGURE 1. Trial design. Acute patients had a second dose injected at the time of response (days 14 or 21). Participation in the follow-up period between 168 and 336 days was optional. IV, intravenously; SC, subcutaneously.*See Methods for exact details of dose regimens.
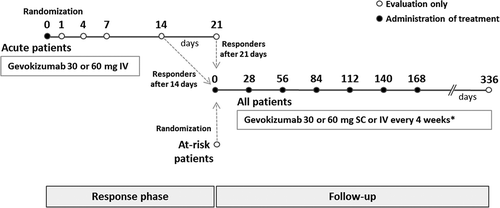
TABLE 1. Baseline characteristics of patients.
TABLE 2. Emergent adverse events most frequently reported during the treatment period in acute and at-risk patients according to system organ classes.
FIGURE 3. Rapid increase in cumulative percentage of responders in 14 acute patients evaluable for treatment response during the response phase.
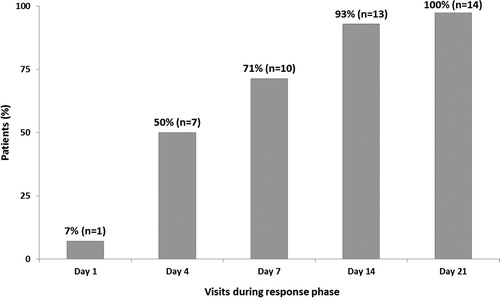
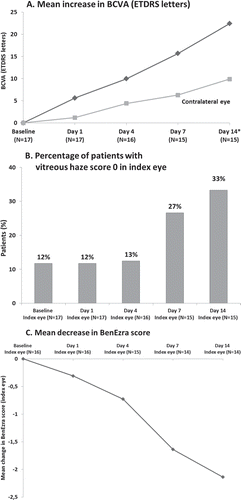
FIGURE 4. Marked improvement of ophthalmologic parameters in acute patients during response phase (including acute patients withdrawn before evaluation of response): (a) BCVA; (b) vitreous haze; and (c) BenEzra score (one eye not evaluated due to high vitreous haze). Data during response phase are provided up to the day 14 visit (all but one patient responded by day 14 although one patient had a supplementary visit to confirm response at day 21).