Figures & data
Table I. Baseline patient characteristics.
Table II. Assisted reproductive technology cycle history.
Figure 1. Most common reasons cited by clinicians for prescribing the 2:1 formulation of follitropin alfa and lutropin alfa. (a) The number of patients who were prescribed the 2:1 formulation of follitropin alfa and lutropin alfa for one (or more) of the pre-specified reasons. (b) The number of patients who were prescribed the 2:1 formulation of follitropin alfa and lutropin alfa for ‘other’ reasons (the seven reasons most frequently cited by clinicians are shown). ART, assisted reproductive technology; LH, luteinizing hormone; PCOS, polycystic ovarian syndrome.
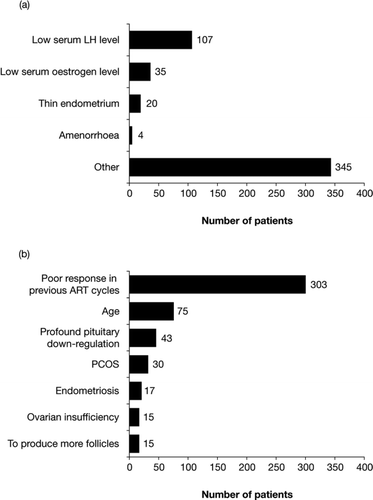
Table III. Concomitant treatments received during assisted reproductive technology cycles.
Table IV. Number of fresh embryos transferred following ovarian stimulation using the 2:1 formulation of follitropin alfa and lutropin alfa.