Figures & data
Figure 1. E. coli clinical isolates stimulating platelet aggregation in plasma. (A) Effect of E. coli CFT073 concentration on the lag time for the onset of platelet aggregation. (A.i) Bacterial suspensions at OD600nm 1.6 were used at different dilutions in platelet aggregation assays as indicated. Platelet-rich plasma (PRP) was 80% of the final volume and was kept constant in all reactions. (A.ii) Lag time for the onset of aggregation was measured in platelets from seven different donors using a 10-fold dilution of the bacterial suspension. Aggregation was observed in six out of seven donors, and lag times are indicated. Experiments were performed on different days (mean ± SD, n = 6). (B) Effect of E. coli RS218 concentration on the lag time for the onset of platelet aggregation. (B.i) Assays were performed as indicated in A.i. for E. coli RS218. (B.ii) Lag time for the onset of aggregation was measured as explained in A.ii. Aggregation was observed in six out of seven donors, and lag times are indicated. Experiments were performed on different days (mean ± SD, n = 6). (C) Effect of crosslinked mAb IV.3 concentration on platelet aggregation. In order to crosslink the FcγRIIA receptor, platelets were pre-incubated for 3 min with 2, 4 or 6 μg/mL mAb IV.3 followed by addition of 15, 30, or 45 μg/mL anti-mouse IgG F(ab’)2, respectively. Platelet-rich plasma (PRP) was 80% of the final volume and was kept constant in all reactions.
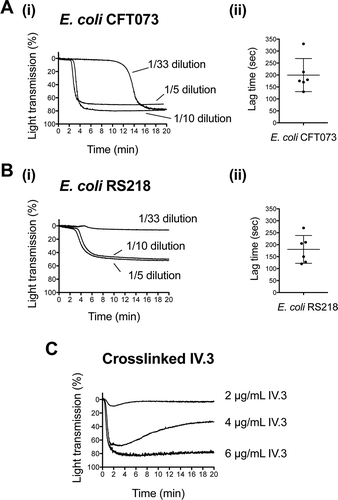
Figure 2. E. coli-induced platelet aggregation in plasma depends on FcγRIIA and Src and Syk tyrosine kinases. (A) Effect of αIIbβ3 antagonist, eptifibatide, and anti-FcγRIIA mAb IV.3 on E. coli CFT073-induced platelet aggregation. PRP was incubated for 2 min with eptifibatide (9 μM) or 10 min with mAb IV.3 (10 μg/mL) or vehicle prior to addition of bacteria, and platelet aggregation was monitored by light transmission aggregometry. The results on the right-hand side graph are shown as mean ± SD of five independent experiments; *p < 0.05. One representative experiment is shown on the left. (B) Effect of αIIbβ3 antagonist, eptifibatide, and anti-FcγRIIA mAb IV.3 on E. coli RS218-induced platelet aggregation. The same experimental conditions as in Figure 2A were used for E. coli RS218. The results on the right-hand side graph are shown as mean ± SD of four independent experiments; *p < 0.05. One representative experiment is shown on the left. (C) Effect of the Src-tyrosine kinase inhibitor, dasatinib, and the Syk-tyrosine kinase inhibitor, PRT-060318, in E. coli CFT073-induced platelet aggregation in plasma. PRP was incubated for 2 min with dasatinib (4 μM) or PRT-060318 (10 μM) or vehicle prior to addition of bacteria, and platelet aggregation was monitored. The results on the right-hand side graph are shown as mean ± SD of four independent experiments; *p < 0.05. One representative experiment is shown on the left. (D) Effect of the Src-tyrosine kinase inhibitor, dasatinib, and the Syk-tyrosine kinase inhibitor, PRT-060318, in E. coli RS218-induced platelet aggregation in plasma. The same experimental conditions as in Figure 2C were used for E. coli RS218. The results on the right-hand side graph are shown as mean ± SD of four independent experiments; *p < 0.05. One representative experiment is shown on the left.
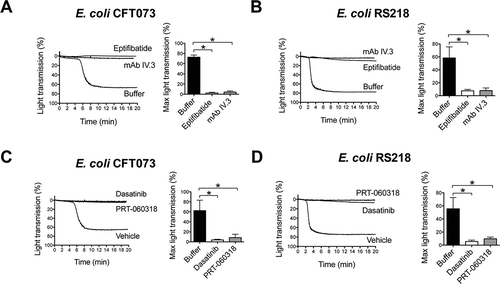
Figure 3. Platelet activation by E. coli requires the interplay between αIIbβ3 and FcγRIIA. (A) αIIbβ3 and FcγRIIA mediate E. coli induced platelet secretion. Platelet-rich plasma was incubated for 2 min with eptifibatide (9 μM) or 10 min with mAb IV.3 (10 μg/mL) or vehicle prior to addition of bacteria, and platelet aggregation was monitored by light transmission aggregometry. Reactions were also performed by crosslinking FcγRIIA receptor. Platelets were pre-incubated for 3 min with 4 μg/mL monoclonal antibody (mAb) IV.3 followed by addition of 30 μg/mL anti-mouse IgG F(ab’)2. Supernatants were collected at time of full aggregation, or a parallel time point in the case of inhibition. Supernatants were analyzed for ATP release by luciferin–luciferase assay. ATP levels released by TRAP (100 μM) stimulated platelets were used to normalize data. The results are shown as mean ± SD of five independent experiments for E. coli CFT073 and RS218 and three independent experiments for crosslinked mAb IV.3; *p < 0.05. (B) E. coli induces tyrosine phosphorylation of FcγRIIA that is dependent on αIIbβ3. Aggregation reactions were performed in plasma in the presence or absence of eptifibatide (9 μM) and cell lysates collected at time of full aggregation, or equivalent times in the case of eptifibatide-treated samples where aggregation was inhibited. Immunoprecipitations for FcγRIIA were performed and tyrosine-phosphorylation detected by Western blot. Representative results of three independent experiments are shown. (C) hIgGs reconstitute aggregation to E. coli in washed platelets. Aggregation reactions to E. coli CFT073 and E. coli RS218 were performed in washed platelets in the presence or absence of IgG-depleted fibrinogen (1 mg/mL) and pooled human IgGs from healthy donors (hIgG) (0.1 mg/mL). Three independent experiments were performed per strain using different platelet donors. One representative experiment is shown for E. coli RS218. For E. coli CFT073, platelet aggregation was restored in two out of three platelet donors when supplementing the reactions with fibrinogen plus hIgG, and one representative experiment is shown.
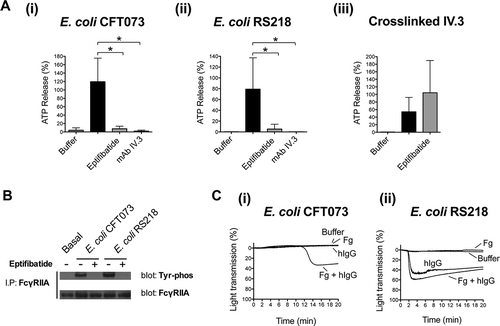
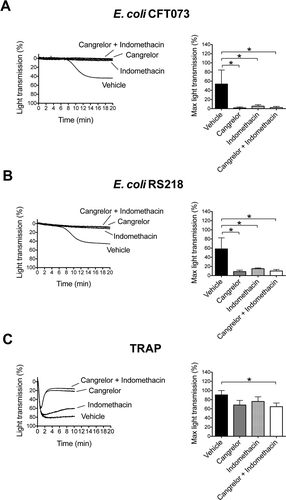
Figure 4. Platelet activation by E. coli depends on secondary mediators ADP and TxA2. Platelet-rich plasma was incubated for 2 min with the cyclooxygendase inhibitor indomethacin (10 μM), ADP-receptor P2Y12 inhibitor Cangrelor (1 μM), or vehicle (DMSO) prior to addition of E. coli CFT073 (A), E. coli RS218 (B), or 50 μM TRAP (C), and platelet aggregation was monitored. The results are shown as mean ± SD of three independent experiments; *p < 0.05.