Figures & data
Figure 1. The sequence of the ectodomain of HIV-1 gp41 a.a. 512-665, gp41e-FP, with underlined Trps. The black bar pointed out the FP region (FP-23, a.a. 512-534), *marked the Trp mutated residue on FP-23 (designated as FP-23-W2) and the arrows indicated the S598C/S604C double mutated positions. Gp41 ectodomain devoid of FP, including a.a. 535–665, was designated as gp41e.

Figure 2. Bis-ANS emission spectra upon addition of gp41e-FP, gp41e or FP-23. Background fluorescence due to the medium has been subtracted. The intensities enhanced by the addition of the peptides were caused by exposure of the hydrophobic binding sites of the peptides and gp41e-FP has the strongest effect. This indicated the prominent contribution of FP region to the hydrophobic surface of gp41 ectodomain.
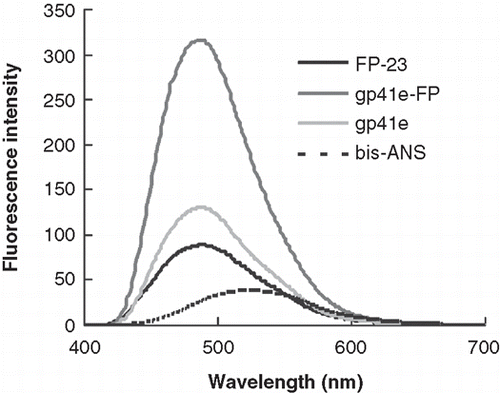
Figure 3. Binding affinity and shedding of gp41e-FP, gp41e or FP-23 to the phospholipid membrane bilayer by SPR measurements. (A) Binding affinity of gp41e-FP, gp41e and FP-23 with raft-liposome. Despite the smaller size, FP-23 associated with the membranes better than gp41e, suggesting the high binding affinity of FP region for the membrane. (B) Dissociation constant (KD) analysed from (A) (black box) and relative shedding of gp41e-FP, gp41e and FP-23 from the DMPC liposomes (grey bar).
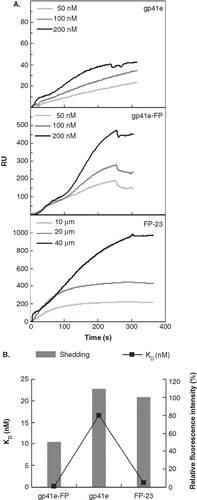
Figure 4. The insertion depth into the membrane measured from NBD labeled peptides quenched by Co2+. (A) The fluorescence emission spectra of NBD-FP-23, NBD-S546C and NBD-S546C-FP in raft-liposome show the higher intensity of the fusion peptide and indicate the strong hydrophobic interaction between membrane and FP region. The larger intensity of NBD-S546C-FP than NBD-S546C signifies that the NHR region is located deeper in the liposome due to the presence of FP, which is thought to penetrate into the membrane. (B) Stern-Volmer plot presents the small KSV (in the parentheses, unit of mM-1) of NBD-FP-23, demonstrating the deeper insertion of FP region into the membrane than gp41 ectodomain devoid of FP. The intermediate KSV value for S546C-FP between FP-23 and S546C concurs with the result displayed in (A). Both data consistently show that the NHR region penetrates deeper into the bilayer by the upstream FP sequence.
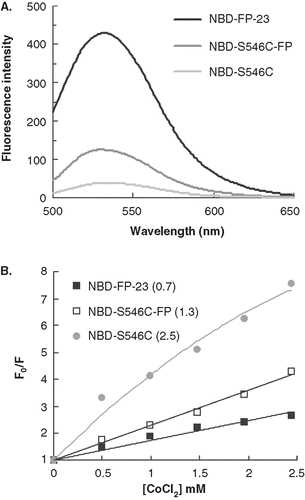
Figure 5. Tryptophan fluorescence emission spectra of gp41e-FP, gp41e and FP-23-W2 in raft-liposome and buffer solution (A) and Stern-Volmer plot and constants KSV (in the parentheses, unit of M-1) quenched by acrylamide (B). A typical maximal fluorescence emission for tryptophan in a polar environment was seen for FP-23-W2 in buffer solution (at 353 nm), and blue shifts were found for all other spectra, indicating the insertion of FP into the membranes and the aggregation of gp41e-FP and gp41e. A large KSV indicates the accessibility of the tryptophan residue for the quencher. Hence the smaller KSV for FP23-W2 than that for gp41e-FP or gp41e argues strongly for the deep immersion of FP in the model membrane.
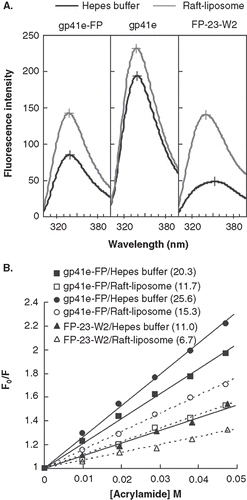
Figure 6. Membrane dehydration as probed by the intensity of carbonyl stretching band of the gp41 fragments in association with DMPC/DMPG lipid bilayer at L/P = 50. The bands at 1742 and 1726 cm-1 arise from dehydrated and hydrated carbonyl stretching, respectively. Higher fraction of dehydrated band for FP-23 and gp41e-FP embedded lipid bilayer compared to the phospholipid alone signifies membrane perturbation by these fragments. In contrast, gp41e devoid of FP causes no change in the hydration state of the phopholipid.
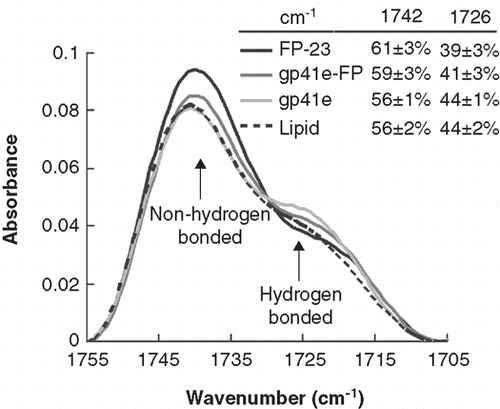
Table I. Insertion angles and tilt angles of lipid acyl chain from analyzing ATR-FTIR spectra of lipid (DMPC:DMPG 1:1) and gp41e-FP, gp41e or FP-23 in the vesicular solution with L/P = 50 at 22°C and pH 7.0a.