Figures & data
Figure 1. (A) Lipid topology in liposome fusion. The simplified schematic depicts the hypothetical lipid arrangements along the reaction pathway from unfused liposomes via the stalk and hemifusion intermediates to complete lipid mixing. (B) Design of LV-peptides used in this study. The hydrophobic cores are flanked by Lys residues and a Trp is added for quantification of peptides.
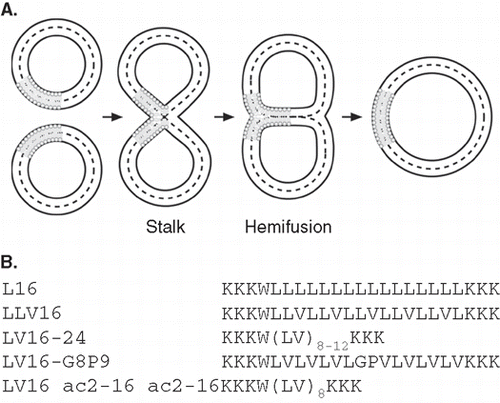
Figure 2. Dependence of liposome fusion elicited by LV-peptides on primary structure. (A) The panels show the kinetics of total fusion, OL mixing, IL mixing, and IL mixing normalized to OL mixing. Data are averages from 4–8 experiments recorded at P/L = 0.0037–0.0061 and scaled to P/L = 0.005 for better comparability. (B) The percentage of hemifusion as a function of reaction time as calculated from total and IL mixing kinetics given in part A. (C) Kinetics of dithionite bleaching. The decrease of NBD-fluorescence upon incubation of donor liposomes with 20 mM Na-dithionite on ice to about two-thirds of the original value after ∼ 20 min indicates preferential bleaching of NBD-PE within the outer leaflet.
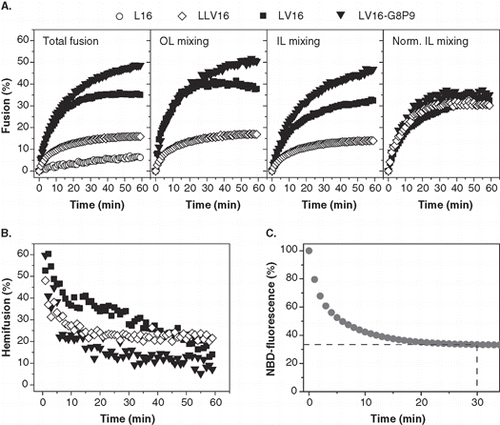
Figure 3. Secondary structure of LV-peptides with different hydrophobic length in liposomal membranes determined by CD spectroscopy. (A) The curve shapes of the CD spectra recorded at at P/L ≈ 0.01 indicate α-helical structures for LV16, LV20, and LV24 and a predominating β-sheet for LV12. Curves are averaged from 2–5 independent measurements. (B) Deconvolution of the spectra quantitates helix and sheet contents. The remaining secondary structure is accounted for by random coil and turn elements.
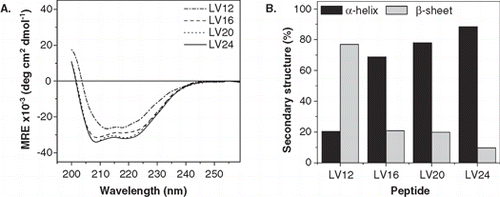
Figure 4. Kinetics of liposome fusion elicited by LV-peptides with different hydrophobic lengths. (A) The panels show the kinetics of total fusion, OL mixing, IL mixing, and IL mixing normalized to OL mixing. Data are averages from 4–8 experiments recorded at P/L = 0.0034–0.0056 and scaled to P/L = 0.005 for better comparability. (B) The initial rates of OL and normalized IL mixing kinetics as obtained by fitting the data presented in part A. (C) The percentage of hemifusion as a function of reaction time as calculated from total and IL mixing kinetics given in part A.
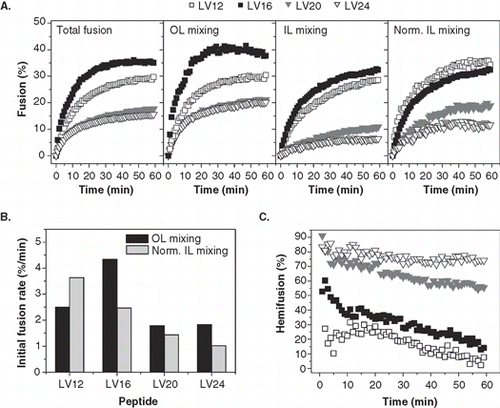
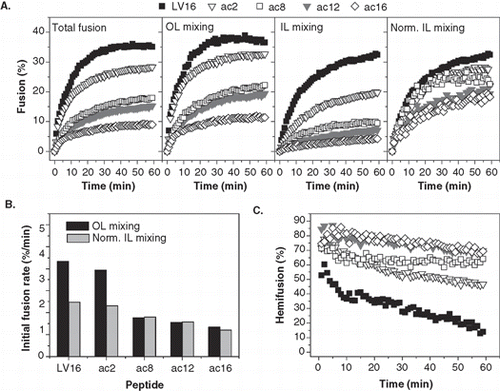
Figure 5. Kinetics of liposome fusion elicited by LV-peptides with covalently attached acyl chains. (A) The panels show the kinetics of total fusion, OL mixing, IL mixing, and IL mixing normalized to OL mixing. Data are averages from 5–8 experiments recorded at P/L = 0.0036–0.0059 and scaled to P/L = 0.005 for better comparability. (B) The initial rates of OL and normalized IL mixing kinetics as obtained by fitting the data presented in part A.