Figures & data
Figure 1. (A) Amino acids sequence alignment analysis of Rab36 from several organisms, showing the N-terminal 66aa extension in human Rab36 was not conserved among divergent organisms. (B) Comparison of the amino acids sequences of Rab36 and Rab34, residues identical were shown in black background. (C) Examination of tissue expression of transcripts of human Rab36 and Rab34 by PCR using multiple tissue cDNA panels, indicating Rab36 and Rab34 have similar tissue expression pattern.
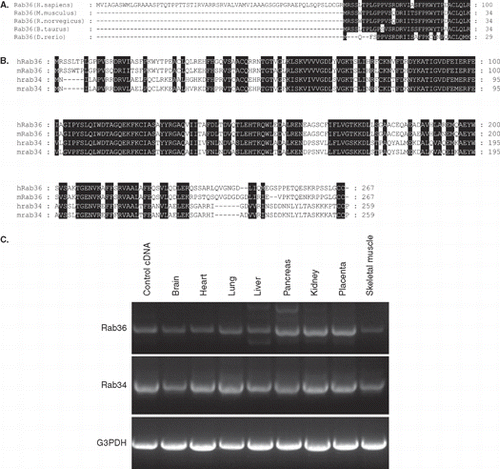
Figure 2. Rab36 is associated with Golgi apparatus. HeLa cells were transfected with EGPF-Rab36WT, and processed for immuno-labeling with antibodies against GM130 to label cis-Golgi, syntaxin 5 to label medium Golgi or TGN46 to label trans-Golgi network. The right panels were magnified to show the co-localization of Rab36 with Golgi apparatus, and demonstrated that Rab36 is preferentially associated with TGN. Bar = 20 μm. This Figure is reproduced in colour in the online version of Molecular Membrane Biology.
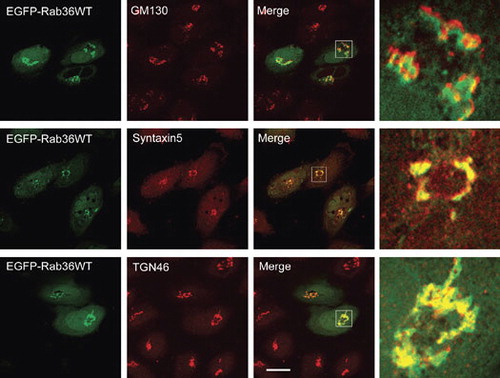
Figure 3. Rab36 regulates peri-nuclear distribution of late endosomes and lysosomes from peripheries. (A) HeLa cells were transfected with EGFP-Rab36WT, and processed for immuno-labeling with antibodies against EEA1, LBPA, CD63 and Lamp1 respectively, showing overexpression of Rab36 induced peri-nuclear distribution of late endosomes and lysosomes marked by LBPA, CD63 or Lamp1, but had no effects on early endosomes marked by EEA1. The inset showed the clustered vesicles not overlapping with Rab36-marked Golgi apparatus. (B) Expression of active mutant form (Q116L) but not negative mutant form (T71N) of Rab36 redistributes late endocytic compartments to peri-nuclear region. (C) The effects of Rab36 on the distribution of late endosomes and lysosomes were further confirmed by over-expressing EGFP-Rab36WT in NRK cells and examined with antibodies against LBPA or Lamp2. The effects of over-expressing Rab34 were also compared. (D) NRK cells expressing EGFP-Rab36WT were treated with nocodazole of 10 μg/ml for 1 hour at 37°C, then processed for immuno-labeling with anti-TGN38 or anti-Lamp2. The result showed that GFP-Rab36 is redistributed to Golgi-fragment, and the late endosomes/lysosomes are dispersed. Bar = 20 μm. This Figure is reproduced in colour in the online version of Molecular Membrane Biology.
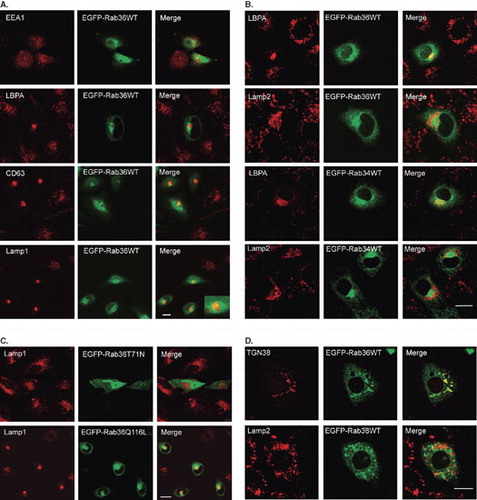
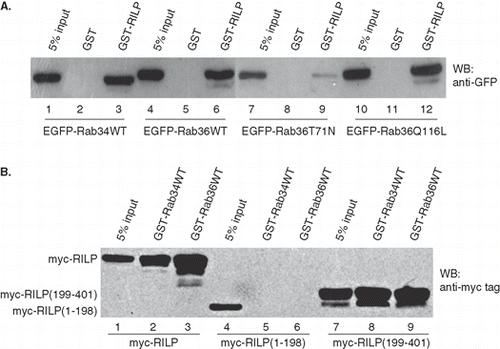
Figure 4. Rab36 interacts with RILP. (A) GST-RILP was immobilized on GST-sepharose beads and used to pull down GFP-Rab proteins from cell lysates containing expressed GFP-Rab36 or Rab34. The results showed RILP specifically interacts with Rab34, Rab36WT or Rab36Q116L, but weakly interacts with Rab36T71N. GST was used for control. (B) Characterization of the region in RILP interacting with Rab36. GST-Rab proteins were immobilized on GST-sepharose beads and used to pull down myc-tagged proteins from cell lysates containing expressed myc-RILP, myc-RILP(1–198) or myc-RILP (199–401). The data further confirmed the interaction between Rab36 and RILP, and indicated both Rab36 and Rab34 interact with C-terminal region of RILP.