Figures & data
Figure 1. Diagram of electron flows generated by Ecto-NOX activity. Electrons can move from inside to outside (in→out) or can be retained in the extracellular space (out→out). The in→out movement is directed from the intracellular NAD(P)H to the inter-membrane quinone (CoQ) and finally to extracellular electron acceptors, such as O2 or protein disulfides. In the out→out movement both the electron donor and acceptor are located outside the cell. NQO1, NAD(P)H:ubiquinone oxidoreductase. Ecto-NOX, hydroquinone (NADH) oxidase.
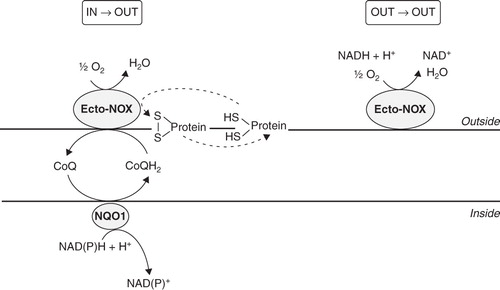
Figure 2. Cofactor specificity and identity of the platelet NAD(P)H oxidase system. (A) NAD(P)H oxidase activity and NAD(P)H-dependent WST-1 reduction. Enzyme activities were measured spectrophotometrically, as reported under Materials and methods. Values are the means of five independent experiments, each performed in triplicate. *p < 0.001 vs. NADH-containing assays. (B) Western blot analysis of Ecto-NOX1 protein. Platelet plasma membranes (PLT) were immunoblotted with anti-Ecto-NOX1 antibody. Positive control is represented by cellular extracts from lymphocytes (Lympho). The arrow points to Ecto-NOX1 band; molecular weight standards are shown on the right. The radiograph is representative of four similar experiments.
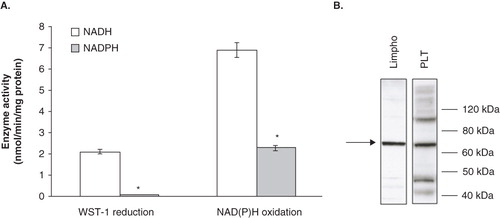
Figure 3. NADH-dependent WST-1 reduction and expression of Ecto-NOX1. (A) Western blot analysis of Ecto-NOX1. Platelets were left untreated or treated with 100 μM capsaicin (Caps) for 45 min, and immunoblotted with anti-Ecto-NOX1 antibody. The second bar represents platelets pre-treated with NAC for 15 min, before incubation with capsaicin; the third bar represents platelets incubated with capsaicin in the presence of 1 mM cycloheximide (CX). The radiograph is representative of four similar experiments. The histogram represents the densitometric analysis of autoradiography. Values are the means ± SE of three independent experiments, values are reported as fold over control, arbitrarily set to 1, after normalization with tubulin. *p < 0.001 vs. untreated platelets; **p < 0.05 vs. capsaicin. (B) NADH oxidation. Platelets were treated as in A and NADH oxidase activity was detected in whole cells, as described in Materials and methods.
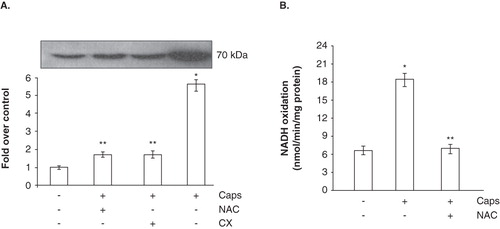
Figure 4. Time course experiment of ROS production by capsaicin. Platelets were incubated with 100 μM capsaicin for the indicated times, before assessing radical formation by flow cytometry. Values are the means ± SE of five independent experiments. Dashed line: control platelets. Solid line: capsaicin-treated platelets.
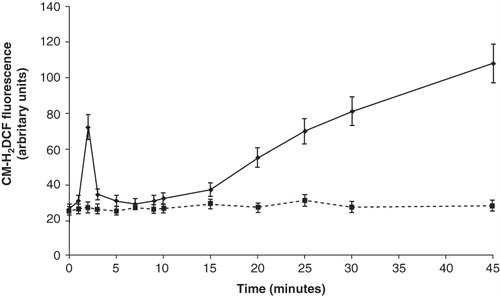
Table I. Capsaicin modifies platelet redox state.
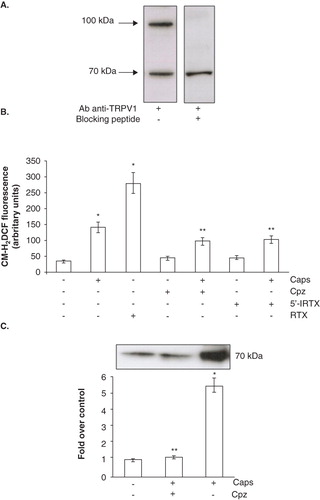
Figure 5. TRPV1 involvement in Ecto-NOX1 modulation. (A) Western blot analysis of TRPV1 receptor. Platelet extracts were immunoblotted with anti-TRPV1 antibody, alone or with TRPV1-specific blocking peptide. The radiographs are representative of four similar experiments. (B) ROS production with TRPV1 agonists and antagonists. Platelets were left untreated or treated with capsaicin (Caps, 100 μM) and resiniferatoxin (RTX, 10 μM) for 45 min; some samples were also pre-treated either with capsazepine (Cpz, 50 μM) or 5′-iodo-resiniferatoxin (5′-IRTX, 20 μM) for 10 min, before incubation with capsaicin. Data are the means ± SE of three independent experiments, each performed in triplicate. *p < 0.001 vs. untreated platelets; **p < 0.05 vs. capsaicin. (C) Western blot analysis of Ecto-NOX1 with TRPV1 agonists and antagonists. Platelets were left untreated or treated with 100 μM capsaicin for 45 min; some samples were also pre-treated with capsazepine for 10 minutes, before incubation with capsaicin. The radiograph is representative of four similar experiments. The histogram represents the densitometric analysis of autoradiography. Values are the means ± SE of three independent experiments, values are reported as fold over control, arbitrarily set to 1, after normalization with tubulin. *p < 0.001 vs. untreated platelets; **p < 0.05 vs. capsaicin.