Figures & data
Figure 1. Chemical structures of various photosensitizing chemical agents. Majority of photosensitizing chemical agents may be classified into four main categories according to their chemical structures, including porphyrins, chlorins, porphycenes, and phthalocyanines. Each PSA features a peak absorbance wavelength, typically in the red light region of the spectrum.
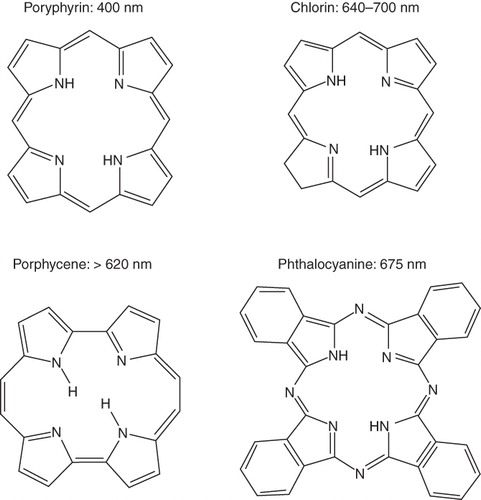
Figure 2. The effects of targeting on the delivery and cytotoxicity of liposome-encapsulated photosensitizers. The addition of targeting ligands to liposomes encapsulating photosensitizers improves cytotoxicity compared to non-targeted, PEG-coated liposomes. Exterior PEG-molecules do not allow for close contact between liposomes and cells, whereas targeted formulations induce binding between the two. This close interaction is required for efficient delivery of short-lived radical oxygen species, which are generated from the light-activated encapsulated photosensitizers.
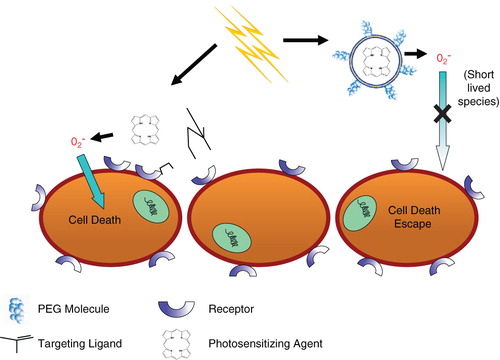
Table I. A partial list of synthetic photo-sensitive phospholipids.
Figure 3. Sites for chemical modifications in phospholipids (photoreactive lipids). Three major parts of phospholipids that can be chemically modified to generate photosensitive molecules. The lipid parts: head group, glycerol backbone and fatty acyl chains are described with their proposed modifications. The references correspond to the currently available designer lipids respectively.
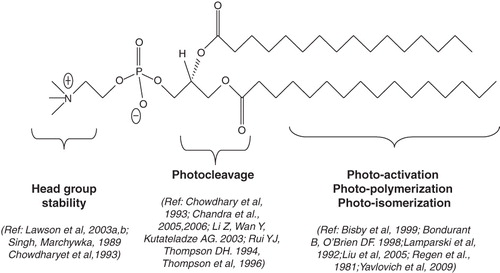
Figure 4. Light sensitive head-group polymerizable lipids. Chemical structures of currently available head group polymerizable lipids.
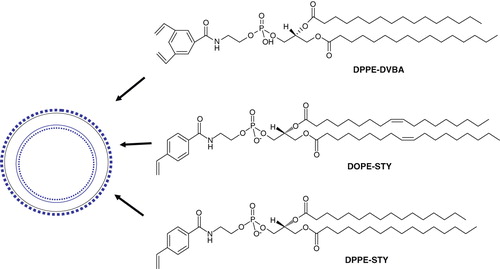
Figure 5. (A) Mechanisms of release of liposome-encapsulated drugs by photo-triggering. The diagram describes various mechanisms of light induced destabilization of liposomes and intracellular localization of released drugs. (B) A cartoon presenting the mechanisms of light triggered release from photo-sensitive liposomes. Two known conformational changes in photo-reactive liposome segments due to light treatment are presented. Light radiation can result in a shrunken domain that leads to leakage between domain boundaries (right, top). Light radiation can also create changes in the liposome bilayer resulting in destabilization and the release of the liposomal contents (right, top).
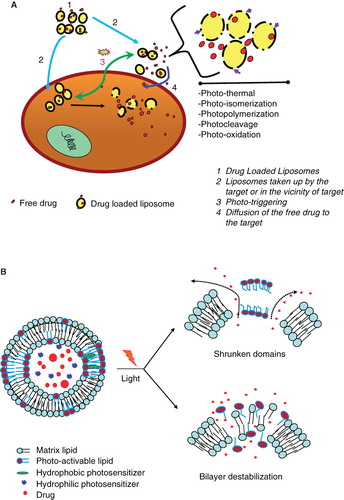
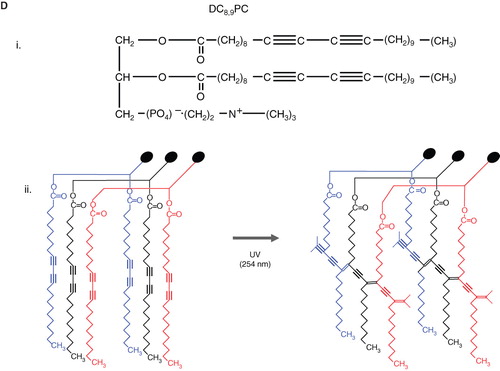
Figure 6. Schematic diagrams of light-mediated chemical reactions in radiation-sensitive lipids. (A) Photo-oxidation of lipids containing vinyl ether linkages by reactive oxygen species generated by a series of photo sensitizers (Adapted from Thompson et al., BBA, Citation1996). (B) Photo isomerization of azobenzene groups undergo wavelength-specific manner radiation leading to cis-trans conformational changes. (Adapted from Morgan et al., FEBS Letters, Citation1995 and Liu et al., BBA, Citation2005). The structrue of photoisomerizable cholesterol is also shown on the right (adapted from Liu et al, Citation2005). (C) Photo cleavable lipids (i–iii); the structure of representative lipids that are subject to cleavage upon light treatment. (iv) The photocleaved breakdown products lead to destabilization of liposome membrane. (D) (i) Chemical structure of a photo-polymerizable phospholipid DC8,9PC. (ii) A cartoon depicting photo-crosslinking of DC8,9PC upon UV treatment. (Adapted from Singh A & Gaber BP, Citation1988).
Table II. A summary of AuNSTs-liposome systems.
Figure 7. Gold nanostuctures-liposome systems. Diagram showing AuNPs entrapment in different regions of liposomes. (a) Hydrophilic Au-MSA in the aqueous core, (b) Hydrophobic Au-C6SH in the lipid bilayer, (c) DPPE-Nanogold® tethered to lipid bilayer.
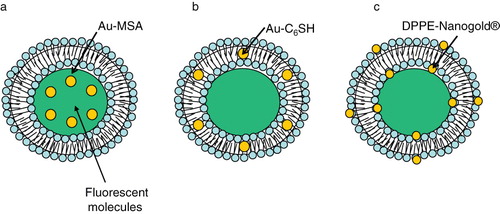
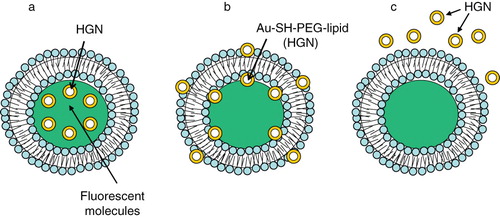
Figure 8. Gold nanostuctures-liposome systems. Diagram showing HGNs entrapment in different regions of liposomes. (a) HGNs in the aqueous core, (b) Au-SH-PEG-lipid tethered to lipid bilayer, (c) HGNs suspended freely outside.