Figures & data
Figure 1. Membrane physical parameters relevant for coat proteins interacting with membranes. (A) Lipid packing defects in an initially flat membrane can be created by a stretching force increasing membrane tension, by membrane curvature or by the presence of conical shaped lipids. (B) Relationships between the various physical parameters relevant for COPI vesicle formation. Global parameters (membrane rigidity and membrane tension, in red) and microscopic parameters (membrane composition and lipid packing, in green) impact on each other and on membrane curvature (in blue). An arrow drawn from parameter X to parameter Y means that the value of X affects Y.
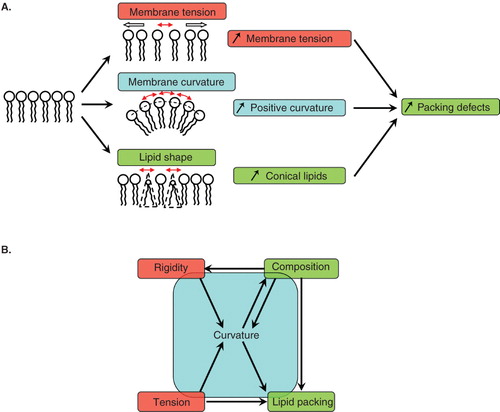
Figure 2. In vitro assays developed to study membrane deformation and fission by coat proteins. Schematic drawings depicting each assay and the effects of coat components (red crescents) binding are shown in the left and middle columns, respectively. (A) Isolated Golgi membranes and small synthetic liposomes. (B) Giant unilamellar vesicles (GUVs) can be aspirated into a micropipette to control and measure membrane tension. Membrane nanotubes are pulled from GUVs either by molecular motors moving on cytoskeletal tracks or by an optical tweezers. Coupling micropipette aspiration with tube pulling by an optical tweezers allows fine tuning of the tube diameter (10–200 nm) via the control of membrane tension and of the force applied by the optical tweezers on the tube. (C) Flat membrane sheets assay. A drop of lipid mix is dried on a glass coverslip. After rehydration, a stack of multilamellar membranes forms with flat sheets at the rims. (D) SUPER (supported bilayers with excess membrane reservoir) templates. 5 μm beads are covered with a lipid bilayer that provides a membrane reservoir. SUPER templates have only been used so far to study the endocytic fission protein dynamin. Note that membrane structure in flat membrane sheets (C) and SUPER templates (D) has not been characterized and the multilayered and folded membranes shown on the drawings only schematically represent the excess membrane reservoir present in these assays. Only the techniques most widely used with each assay are indicated in the column on the right. Note that electron microscopy can also be performed on GUVs, flat membrane sheets or SUPER templates.
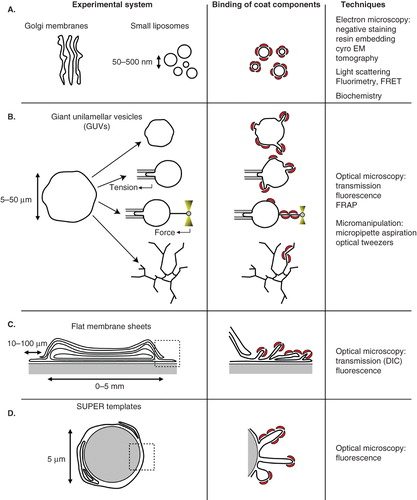
Figure 3. Role of lipid domains in COPI coat assembly, vesicle budding and fission. (A) The COPI coat preferentially assembles on Ld domains of a phase separated membrane exhibiting distinct Ld (green) and Lo (red) domains. Whether such domains exist on Golgi membranes is not clear, especially at the cis-Golgi where the concentration of sphingolipids is very low. After initial recruitment of COPI components in Ld domains, COPI bends the membrane into a bud. If the size of the Ld domain corresponds to a 50–60 nm bud, line tension between the Ld bud and the Lo flat membrane could facilitate membrane fission. (B) On a homogeneous membrane containing Ld and Lo lipids, COPI components interact preferentially with Ld lipids and cluster them in the nascent bud. Local enrichment of Ld lipids in COPI-coated areas may be enhanced by Arf1 dimerization. Positive curvature during budding further sorts Ld lipids in the COPI bud. Line tension between the Ld enriched bud and the comparatively Ld depleted donor membrane could help fission. As suggested in vitro and in clathrin-independent endocytosis, actin polymerization could participate in phase separation and trigger fission. Note that only Arf1 and coatomer are represented as coat components for clarity.
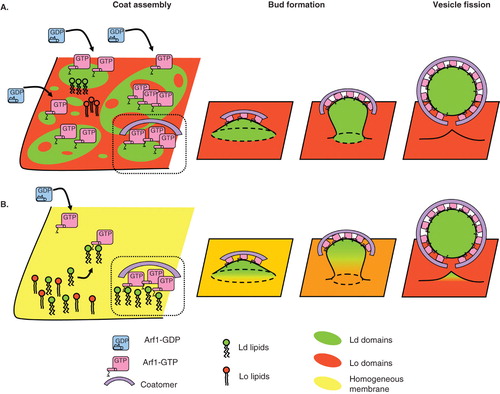
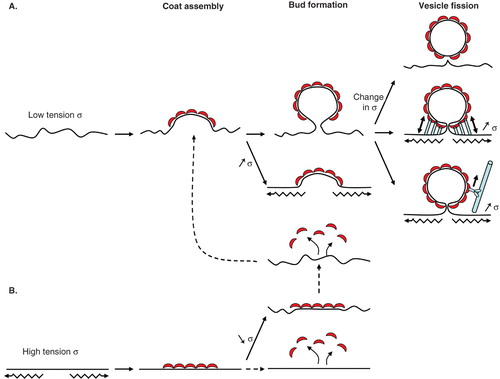
Figure 4. COPI vesicle formation is regulated by membrane tension. (A) At low membrane tension (low σ), the COPI coat assembles with its preferred curved geometry and bends the membrane. From this point, an increase in membrane tension should prevent further deformation. If membrane tension stays low or drops, the COPI coat moulds the membrane into a bud. Tension could then participate in the final fission step. A sudden change in tension (either rise or drop) could destabilize the bud and facilitate fission. Actin polymerization or molecular motors moving on cytoskeletal elements (myosins on actin or kinesins/dynein on microtubules) and interacting with coat proteins could stretch the membrane in the bud neck, increase its tension and trigger fission. (B) At high membrane tension (high σ), the coat may not able to assemble in its curved geometry and form flat lattices. If tension stays high or increases further, the coat may disassemble in an unproductive round of COPI assembly. If tension drops, the coat probably stays in its flat geometry and has to disassemble completely before reassembling into a curved geometry. Dotted lines denote more hypothetical steps.