Figures & data
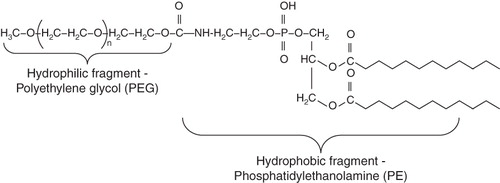
Figure 1. Pharmaceutical micelles. Spontaneous micelle formation from amphiphilic molecules in aqueous media and loading with hydrophobic drug (A), a multifunctional pharmaceutical micelle containing; (a) specific targeting ligand, usually a monoclonal antibody such as 2C5, attached to the micelle surface [Gao et al. Citation2003]; (b) heavy metal atom such as 111In, or Gd loaded onto the micelle via the micelle-incorporated chelating moiety for gamma- or MR imaging application [Torchilin Citation2001]; (c) cell-penetrating peptide, CPP, such as TATp attached to the micelle surface and allowing for the enhanced uptake by the cells [Sawant and Torchilin Citation2009] (B).
![Figure 1. Pharmaceutical micelles. Spontaneous micelle formation from amphiphilic molecules in aqueous media and loading with hydrophobic drug (A), a multifunctional pharmaceutical micelle containing; (a) specific targeting ligand, usually a monoclonal antibody such as 2C5, attached to the micelle surface [Gao et al. Citation2003]; (b) heavy metal atom such as 111In, or Gd loaded onto the micelle via the micelle-incorporated chelating moiety for gamma- or MR imaging application [Torchilin Citation2001]; (c) cell-penetrating peptide, CPP, such as TATp attached to the micelle surface and allowing for the enhanced uptake by the cells [Sawant and Torchilin Citation2009] (B).](/cms/asset/e3dfb339-7895-4529-9c6d-01739f511b1a/imbc_a_516276_f0001_b.jpg)
Table I. Examples of various drug-loaded polymeric micelles.
Table II. Particle diameter and CMC values of PEG-PE micelles.
Figure 3. Schematics of possible ways for PEG-PE-based micellar nanocarrier-mediated cancer therapy. Following the accumulation in a tumour due to EPR effect, micelles can be taken up by cells via endocytosis and release drug. In case of photodynamic therapy (PDT) micellar photosensitizer exerts its cytotoxic effect due to generation of reactive oxygen species (ROS) upon activation at a specific region (tumour) with a laser. Micellar nanocarriers can be made ‘targeted’ by attaching tumour cell-specific ligands to their surface.
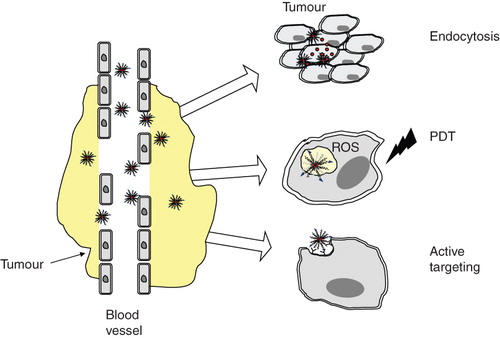
Figure 4. Fluorescence microscopy of the binding of rhodamine-PE-labeled paclitaxel-loaded PEG-PE-based micelles to murine LLC and EL4 cells and to human BT-20 and MCF-7 cells. Cells were grown on cover slips to a confluence of 60–70%, incubated with various preparations for 1 h, washed and mounted cell-side down on glass slides using a fluorescence-free glycerol-based Trevigen® mounting medium. For each pair, left images – phase contrast; right images – fluorescence. Modified from Torchilin (Citation2005a).
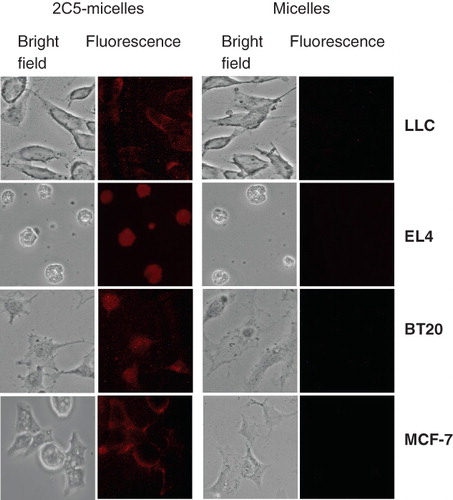
Figure 5. Nuclear fragmentation following PDT of LLC cells with TPP-loaded PEG-PE micelles. An apoptotic cell is indicated by an arrow. Cells were stained with DAPI for visualization of nuclear fragmentation by fluorescence microscopy. Modified from Roby et al. (Citation2006).
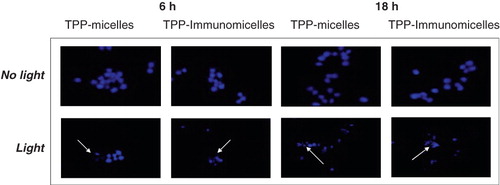
Figure 6. Microscopy of BT-20 cells incubated with PEG-PE/ paclitaxel micelles and PEG-PE/LL/paclitaxel micelles for 2 and 4 h. Bright-field (left images in each pair) and fluorescence (right images in each pair). Arrows indicate fluorescent endosomes in cells incubated with PEG-PE/paclitaxel micelles for 2 h; partially degraded endosomes in cells incubated with PEG-PE/LL/paclitaxel micelles for 2 h; punctuate fluorescent structures in cells incubated with PEG-PE/LL/paclitaxel micelles for 4 h; larger (fused) endosomes in cells incubated with PEG-PE/paclitaxel micelles for 4 h. Modified from Torchilin (Citation2005a).
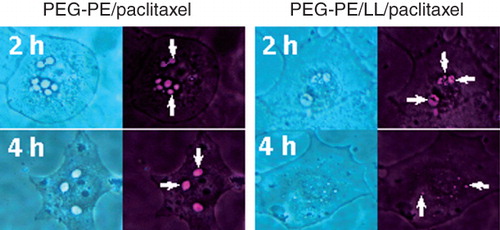
Figure 7. Fluorescence microscopy of the interaction of rhodamine-PE-labeled micelles with BT-20 cells. Arrows indicate binding of micelles to the cell surface (a); formation of endosomes (b); endosomal escape (c) and accumulation in the perinuclear space (d). Modified from Torchilin (Citation2005a).
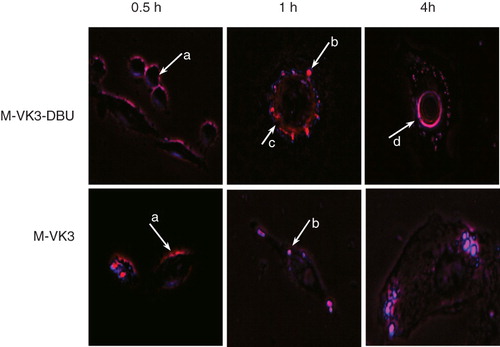
Figure 8. Schematic representation of the TATp-modified micelles (A); In vitro interaction of rhodamine-PE labeled PEG-PE micelles with 4T1 cells. Left panel shows the bright field and right panel shows the fluorescent microscopy of 4T1 cells treated with rhodamine-labeled PEG750-PE micelles (a), rhodamine-labeled PEG750-PE micelles modified with TATp-PEG1000-PE (b). Magnification ×40 objective. Modified from CitationSawant and Torchilin (Citation2009).
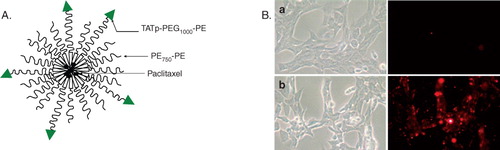
Figure 9. Detection of apoptotic cells by fluorescence microscopy of frozen tumour sections. Apoptosis was determined by TUNEL. The left panel shows the sections stained with DAPI and the right panel shows TUNEL. Negative control (A), free paclitaxel (B), paclitaxel-loaded micelles without TATp (C), paclitaxel-loaded micelles with TATp (D). Magnification ×20 objective. Modified from CitationSawant and Torchilin (Citation2009).
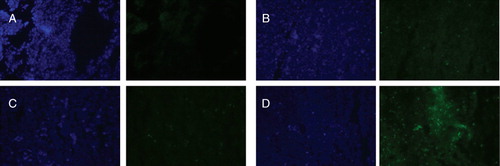
Figure 10. Schematic of a ‘smart’ nanocarrier with a temporarily ‘hidden’ function, for example a CPP, and ‘shielding’ polymeric coat (with or without targeting antibody attached to it) providing longevity in the blood and specific target (tumour) accumulation and preventing the hidden function from premature interaction with target cells. Polymeric chains are attached to the carrier surface via low pH-degradable bonds. After the accumulation in the tumour due to PEG (longevity) and/or antibody (specific targeting), pH-dependent de-shielding of the temporarily hidden cell-penetrating function allow for carrier penetration inside tumour cells.
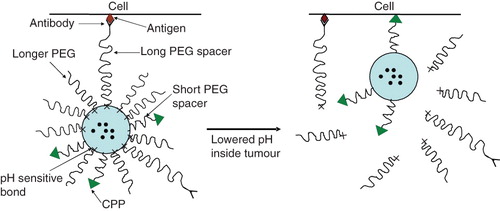
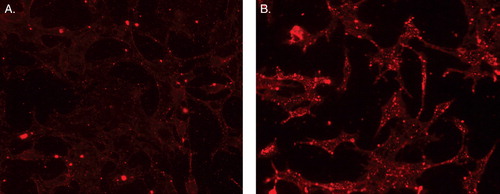
Figure 11. Fluorescence microscopy showing internalization of rhodamine-PE-labeled-TATp-containing micelles by NIH 3T3 fibroblast cells after preincubating micelles at pH 8.0 (A) and pH 5.0 (B)* for 30 min. *The pH of these formulations was raised back to pH 7.4 after their incubation at pH 5.0 and prior to incubation with cell. Modified from Sawant et al. (Citation2006).