Figures & data
Figure 1. A current estimate of the eukaryotic phylogenetic tree. The scheme is a consensus of recent phylogenetic and phylogenomic analyses [Burki et al. Citation2007, Citation2008, Citation2009, Hampl et al. Citation2009, Baurain et al. Citation2010, Glücksman et al. Citation2010, Parfrey et al. Citation2010, Yabuki et al. Citation2010]; relationships with inconsistent support are indicated by dashed branches. The limited molecular data available for Collodictyonida, Ancyromonadida, and Micronuclearia podoventralis attest to their independence on other lineages, but do not allow for their more precise placement in the tree. Following a recent proposal [Cavalier-Smith Citation2010b], the name ‘Chromista’ is here applied in a broader sense as compared to the original meaning of the name; the newly defined Chromista comprise ‘chromalveolates’ expanded by inclusion of Rhizaria and some minor previously unplaced lineages (centroheliozoans and telonemids). Three alternative positions of the root of the tree discussed in the text are marked by wedges. Note that the root R1 as indicated in the tree only approximately corresponds to the ‘unikont-bikont’ rooting previously advocated by Stechmann and Cavalier-Smith (Citation2003) and Richards and Cavalier-Smith (Citation2005), since the latter assumed Apusomonadida to be ‘bikonts’, while more recent evidence and the present scheme shows these two groups branching off on the ‘unikont’ side. Two alternative scenarios on the evolutionary path of the GTPase RAB24 implied by the alternative roots R2 and R3 are shown by green and red arrows, respectively (a scenario for the R1 root is omitted for clarity). Note that the R2 scenario assumes the presence of RAB24 at the root (i.e., already in the LECA) and secondary loss in the Discoba lineage (among other lineages), whereas the R3 scenario implies post-LECA emergence of RAB4 and its primary absence in the Discoba.
![Figure 1. A current estimate of the eukaryotic phylogenetic tree. The scheme is a consensus of recent phylogenetic and phylogenomic analyses [Burki et al. Citation2007, Citation2008, Citation2009, Hampl et al. Citation2009, Baurain et al. Citation2010, Glücksman et al. Citation2010, Parfrey et al. Citation2010, Yabuki et al. Citation2010]; relationships with inconsistent support are indicated by dashed branches. The limited molecular data available for Collodictyonida, Ancyromonadida, and Micronuclearia podoventralis attest to their independence on other lineages, but do not allow for their more precise placement in the tree. Following a recent proposal [Cavalier-Smith Citation2010b], the name ‘Chromista’ is here applied in a broader sense as compared to the original meaning of the name; the newly defined Chromista comprise ‘chromalveolates’ expanded by inclusion of Rhizaria and some minor previously unplaced lineages (centroheliozoans and telonemids). Three alternative positions of the root of the tree discussed in the text are marked by wedges. Note that the root R1 as indicated in the tree only approximately corresponds to the ‘unikont-bikont’ rooting previously advocated by Stechmann and Cavalier-Smith (Citation2003) and Richards and Cavalier-Smith (Citation2005), since the latter assumed Apusomonadida to be ‘bikonts’, while more recent evidence and the present scheme shows these two groups branching off on the ‘unikont’ side. Two alternative scenarios on the evolutionary path of the GTPase RAB24 implied by the alternative roots R2 and R3 are shown by green and red arrows, respectively (a scenario for the R1 root is omitted for clarity). Note that the R2 scenario assumes the presence of RAB24 at the root (i.e., already in the LECA) and secondary loss in the Discoba lineage (among other lineages), whereas the R3 scenario implies post-LECA emergence of RAB4 and its primary absence in the Discoba.](/cms/asset/1cf485f6-9152-4e10-8980-4dbf501df9a8/imbc_a_521201_f0001_b.jpg)
Figure 2. Examples of evolutionary events affecting the ES-associated machinery in the eukaryotic ‘supergroup’ Opisthokonta. The scheme shows a non-exhaustive selection of events with a well-understood relation to the organismal phylogeny. The events are mapped on a schematic opisthokont phylogeny according to the following published surveys: SNARE proteins (Vam7, Npsn, Sec22, Syx6, Syx7, SN47, and Gs15) – Kloepper et al. (Citation2008), Kienle et al. (Citation2009), Sec2 – Elias (Citation2008), BBSome – Hodges et al. (Citation2010), GGA, Epsin, Eps15, caveolins, stonins – Field et al. (Citation2007), ESCRT 0 – Leung et al. (Citation2008), RAB7 – Mackiewicz and Wyroba (2007). The change affecting Vps51 is reported below in this review, the remaining events in the fungal branch are shown according to analyses reported in Ma et al. (Citation2009). The fate of RAB40 and AP-4 in metazoans is based on my own BLASTP searches against the non-redundant protein database at NCBI.
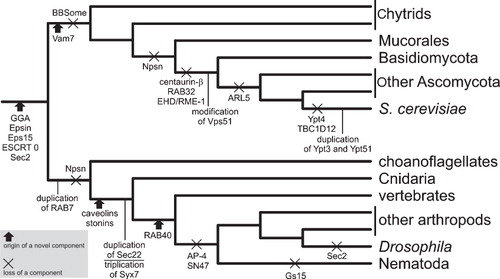
Figure 3. Ancestral RAB paralogs exhibit different propensity to generating lineage-specific in-paralogs. The tree was inferred using the maximum likelihood method (WAG + Γ + I substitution model) as implemented in RAxML-HPC (7.2.6) run at the CIPRES portal (http://www.phylo.org/portal2/). Bootstrap values (based on 100 replicates) only higher than 50 are shown. Note the lack of significant bootstrap support for the RAB11 clade, probably due to the inclusion of several rather divergent sequences (exhibiting long branches in the tree). Sequences selected for the analysis come from nine phylogenetically diverse eukaryotes (Homo sapiens, the chytrid fungus Spizellomyces punctatus, the amoebozoan Dictyostelium discoideum, the heterolobosean Naegleria gruberi, the metamonad Trichomonas vaginalis, the moss Physcomitrella patens, the green alga Chlamydomonas reinhardtii, the oomycete Phytophthora sojae, and the haptophyte Emiliania huxleyi) and represent members (in-paralogs) of three particular ancestral paralogous RAB groups (RAB11, RAB18, RAB23; the assignment of the sequences is based on large-scale phylogenetic analyses of the RAB family, M. Elias, J. B. Dacks, M. C. Field, unpublished work). Sequences from the same species are highlighted in the same colour. The sequence identifiers provided refer to the NCBI protein database (http://www.ncbi.nlm.nih.gov/), except Phytophthora and Spizellomyces, where the sequences come from the JGI (http://genome.jgi-psf.org/Physo1_1/Physo1_1.home.html) and Broad Institute (http://www.broadinstitute.org/annotation/genome/multicellularity_project/MultiHome.html) databases, respectively.
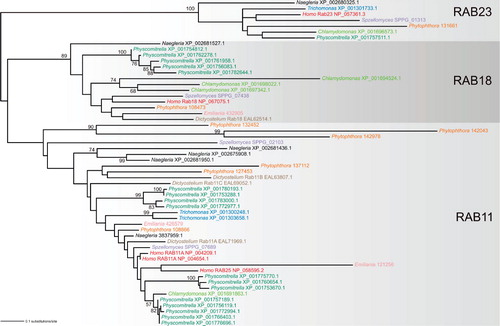
Figure 4. A multiple alignment of the Vps51 domain sequences of putative Vps51 orthologs. The Vps51 domain of representative sequences identified by PSI-BLAST and BLASTP searches with the yeast Vps51p (NP_012945) and the zebrafish Ffr (NP_001036200), respectively, as a query (see text for details), were aligned using the hmmalign program of the HMMER 2.3.2 package and the Pfam Vps51 (PF08700) profile alignment as a template. Sequence IDs correspond to the NCBI protein database, with the exception of the sequences from Batrachochytrium, Mucor, and Emiliania taken from the respective databases at JGI (http://genome.jgi-psf.org/Batde5/Batde5.home.html, http://genome.jgi-psf.org/Mucci2/Mucci2.home.html, http://genome.jgi-psf.org/Emihu1/Emihu1.home.html).
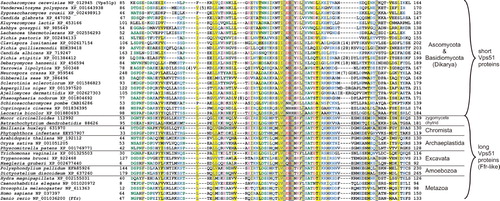
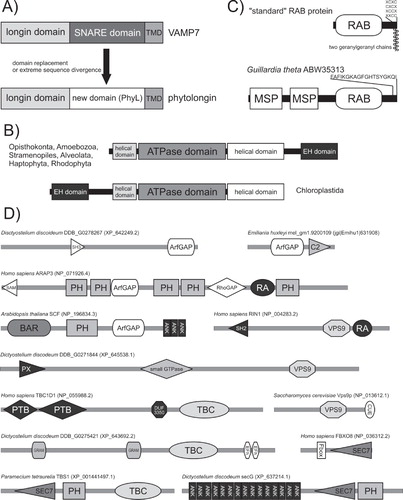
Figure 5. Domain architecture of the membrane-trafficking machinery. (A) Origin of the land plant-specific phytolongin proteins from a SNARE protein of the VAMP7 family via replacement or extreme sequence divergence of the SNARE region (TMD – trans-membrane domain). Adopted from Vedovato et al. (Citation2009). (B) Two different domain architectures of the EHD/RME-1 protein family. (C) Sequence features and domain organization of ‘standard’ RAB proteins and a unique RAB from the cryptomonad Guillardia theta. Note the two geranylgeranyl moieties attached to two cysteine residues occurring (in various arrangements) near the C-terminus of the standard RABs. The Guillardia RAB lacks these C-terminal cysteine residues and instead has an N-terminal extension with a pair of MSP domains. (D) The diversity of taxon-specific domain architectures of proteins serving as regulators of RAB and ARF GTPases. The cartoon shows examples of proteins with ArfGAP, SEC7 (= ArfGEF), TBC (= RabGAP), or VPS9 (= GEF for RAB5-related RABs) domains combined with any of an array of ‘promiscuous’ domains reoccurring in many other proteins. The domain architecture of the proteins shown was determined using SMART (http://smart.embl-heidelberg.de/), Pfam (http://pfam.sanger.ac.uk/search), and CDD (http://www.ncbi.nlm.nih.gov/cdd) search tools. Sequence IDs correspond to the NCBI protein database, with the exception of the protein from Emiliania huxleyi taken from the Emiliania JGI genome database (http://genome.jgi-psf.org/Emihu1/Emihu1.home.html).