Figures & data
Figure 1. Model for topical delivery of CPP-coated NLC delivery approach. (I) Structures of NLC with and without surface modifications. (a) Structure of NLC; (b) Structure of CPP-coated NLC (NLC-CPP); (c) Structure of non-transduction peptide, YKA, coated NLC (NLC-YKA). (II) Various pathways for the entry of nanoparticles into the skin. (a) This panel shows that due to occlusive properties, NLC particles entering into the stratum corneum via the paracellular route are unable to cross SC; (b) Surface modification of NLC particles with CPP favours permeation of NLC into the SC and subsequently into the epidermis; (c) YKA coating on NLC prevents entry of nanoparticles into the skin, (d) NLC and NLC-CPP enter the skin via hair follicle route. (III) An expanded view of the SC and NLC penetration mechanisms (see legend to , part II for description).
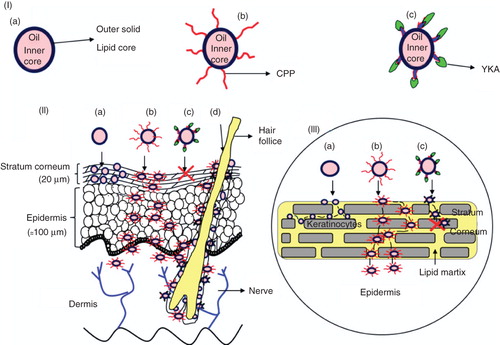
Table I. Examples of different cell-penetrating peptides (CPPs) and their sequences.
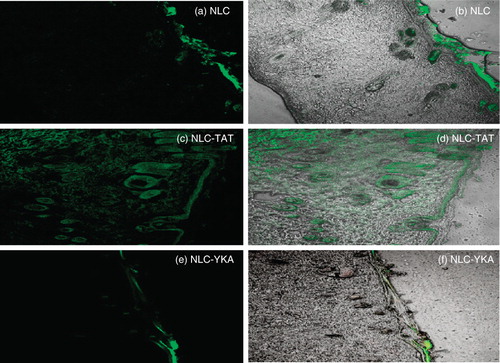
Figure 2. Topical delivery of CPP-coated NLC in rat skin: Lipophilic fluorescent dye (DID) was encapsulated in various NLC preparations and the particles were incubated with rat skin in vitro. The vertical skin sections with a thickness of 30 μm were made with cryotome and were observed under confocal microscope for skin associated fluorescence. Left panel, DID fluorescence; right panel, overlay of bright field and fluorescence. Results indicate that surface modification of nanoparticles with TAT enhances the skin permeation, whereas addition of non-transduction peptide (YKA) inhibits the entry of nanoparticles into the skin.