Figures & data
Figure 1. Model of (A) anterograde and (B) retrograde trafficking pathways between the secretory and endocytic pathway. Clathrin/AP-2-coated endocytic vesicles traffick between the plasma membrane and early endosomes (EE). At the trans-Golgi network (TGN), proteins are incorporated into distinct carriers coated with either clathrin/AP-1A or clathrin/GGAs or AP-3 to be transported to the endosomal system. In polarized epithelial cells, clathrin/AP-1B-coated carriers forming at the TGN and/or recycling endosomes (RE) transport their cargos to the basolateral plasma membrane. On early endosomes, AP-3 coats select their cargos for subsequent transport to late endosomes (LE), a step of membrane traffic, which involve a maturation process including the formation of multivesicular bodies. On early endosomes, the retromer complexes transport proteins back to the TGN. On late endosomes, some proteins are retrieved to the TGN via Rab9 and TIP47 positive carriers.
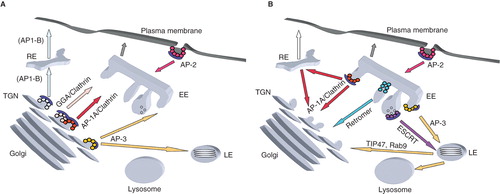
Figure 2. Model of clathrin/AP-1-coated carrier formation on TGN membranes. ARF1, PI-4-P and sorting motifs in cargo tails recruit AP-1 and clathrin to specific membrane subdomains inducing membrane curvature. Actin nucleation complexes associate with CHC at the edges of the clathrin coats. These complexes activate the Arp2/3 complex and trigger actin polymerization, thereby providing the force necessary to initiate carrier tubulation. Tubulated membranes may then recruit BAR domain-containing proteins that interact with N-WASP to sustain further actin polymerization via Arp2/3. Inhibitors of actin polymerization (HIP1R) which bind to clathrin light chains may prevent actin polymerization on the surface of the clathrin coats. Gadkin links AP-1 coat with kinesins and with microtubules. Carrier fission is induced by dynamin linked to the actin cytoskeleton. Thus, actin and microtubules may provide the forces necessary during the late stages of tubule formation, fission and subsequent microtubule based transport.
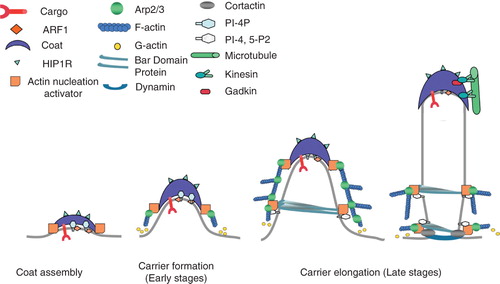
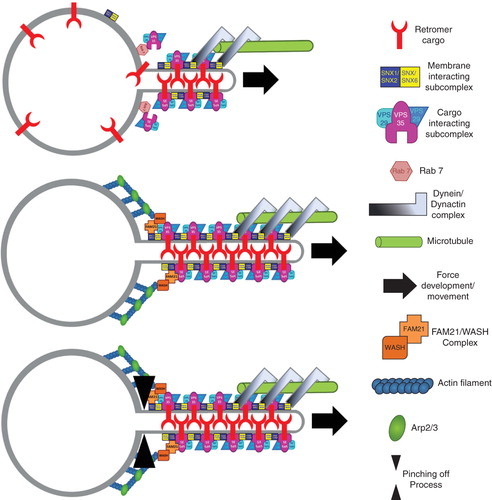
Figure 3. Model for retromer mediated cargo selection and carrier formation. (A) The GTPase Rab 7 recruits the cargo selective subcomplex of the retromer formed by VPS26, VPS29 and VPS35 onto the endosomal membrane where it can engage with its cargo. The membrane interacting subcomplex formed by combinations of SNX1/SNX2 and SNX5/SNX6 is associated via its PI3P binding PX domains with the endosomal membrane. Association of the cargo selective with the membrane interacting subcomplex and possibly multimerisation of the two complexes could lead to the formation of a tubular subdomain of the endosomal membrane, for which the curvature sensing/inducing bar domains of the SNXes are thought to be crucial. Formation of a tubular domain is likely to be assisted by force development achieved through the coupling of the minus end directed dynein/dynactin motor complex to the retromer via SNX5/SNX6. (B) The WASH/FAM21 complex is recruited by the retromer followed by actin polymerisation and Arp2/3 complex mediated actin filament branching which could assist force development. Currently it is unclear how recruitment of dynamin or a dynamin like factor to accomplish membrane fission of the formed carrier is regulated (C).