Figures & data
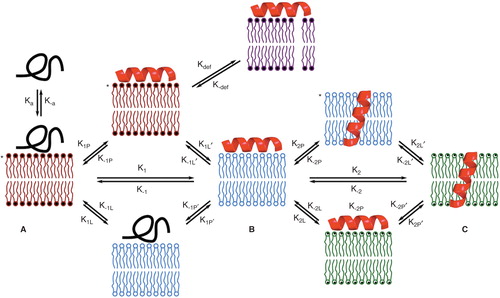
Figure 1. Diffusion processes of membrane molecules: transverse diffusion (interleaflet exchange; flip-flop), lateral diffusion (characterized by the coefficient DL) and rotational diffusion (characterized by the coefficient DR). This Figure is reproduced in color in Molecular Membrane Biology online.
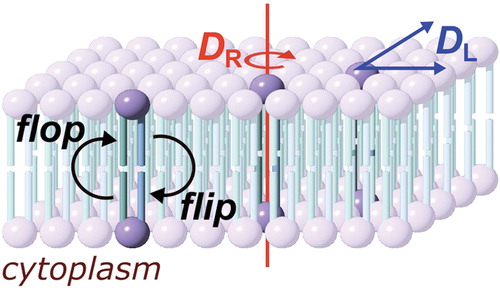
Figure 2. Methods for the preparation of supported lipid bilayers (SLBs). (A) In the Langmuir-Blodgett (LB) method, the solid substrate is drawn through a monolayer of one lipid (lipid A) and subsequently pushed through a second layer (lipid B), producing an asymmetric layer. Each monolayer is at a controlled area per lipid molecule and surface pressure, giving excellent control of the composition of the SLB; (B) Vesicle fusion (VF) is the simplest method, but is not useful for the preparation of asymmetric bilayers; (C) In the Langmuir-Schaeffer (LS) method, entire intact monolayers are transferred to the solid substrate in successive operations; (D) Hybrid LS/VF or LB/VF methods. These allow asymmetric bilayers to be prepared in situ and are ideal for conducting measurements on SLBs immediately after preparation; (E) Tethered or polymer-supported bilayers consist of an amphiphile anchored to the surface of the substrate by a polymer (e.g., polyethyleneglycol), around which the proximal monolayer is formed. Tethered bilayers have a greater water layer depth between the proximal surface of the bilayer and the solid support. This Figure is reproduced in color in Molecular Membrane Biology online.
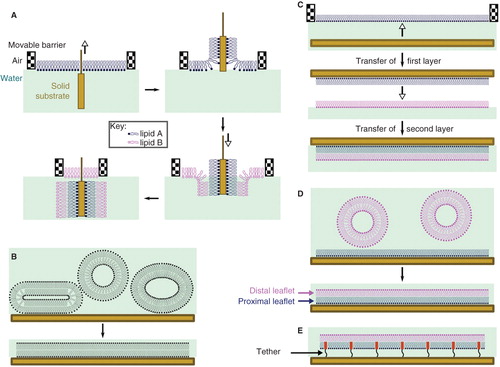
Figure 3. Models for the mode of action of antimicrobial peptides. (A) Toroidal pores, stabilized by the peptide, consisting of a lipid-lined pore and contiguous membrane leaflets; (B) Membrane thinning, in which peptide binding leads to a reduction in the thickness of the bilayer. The thinner bilayer presents a reduced barrier to the flip-flop of lipids between leaflets, as well as to the formation of transient defects; (C) Defect-mediated poration, in which the formation of transient defects (that are significantly less hydrated than toroidal pores) is promoted following peptide binding; (D) The barrel-stave model, consisting of a peptide-lined pore; (E) The carpet model, in which bilayer integrity is disrupted by the formation of peptide aggregates; (F) Detergent models, in which membrane disruption occurs through the formation of peptide-lipid micelles that remove lipids from the membrane. This Figure is reproduced in color in Molecular Membrane Biology online.
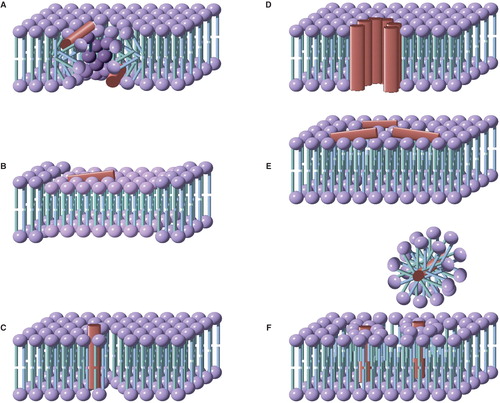
Figure 4. A model for peptide-lipid interactions that incorporates the kinetics of both peptide and lipid-based transformations. Forward processes involving peptides and lipids have ‘P’ and ‘L’ subscripts respectively. Intermediate A is formed immediately following peptide binding. As shown here, the unfolded peptide initially binds, as occurs in many (but not all) cases. Adoption of peptide 2° structure leads to intermediate B following lipid relaxation. Intermediate B in many systems corresponds to a bilayer of reduced thickness following thinning. Intermediate C could correspond to the system following peptide insertion (as shown), but in a general scheme C would correspond to any system that involves a peptide rearrangement from B, followed by lipid relaxation. Processes such as defect formation (represented by kdef) will be favored in those intermediates (indicated by asterisks) in which the lipid component of the system is not at equilibrium. This Figure is reproduced in color in Molecular Membrane Biology online.