Figures & data
Figure 1. Asymmetry, symmetry and the effects of single cysteine mutations on transport activity of the bovine 2-oxoglutarate carrier OGC. The backbone is shown in yellow and is based on the structure of the bovine AAC1 (Pebay-Peyroula et al. Citation2003). The conservation and average symmetry scores of the OGC subfamily are represented by the size and colour of the Cβ atom, respectively. Large spheres indicate residues that are well-conserved in the subfamily of OGC, whereas small spheres are not. Asymmetric residues are shown in a colour scale from red (highly asymmetric) to white (neutral) (A), whereas symmetric residues are shown in a colour scale from blue (highly symmetric) to white (neutral) (C) (Cappello et al. Citation2006). The asymmetric (B) and symmetric residues (D) were also coloured according to the relative initial transport velocity of the single cysteine mutant proteins compared with the wild-type at the external substrate concentration equal to the km of the wild-type: red, 0–15%; orange 16–50%; green, 51–100%; white, no data. The black encircled residues are the three contact points of the substrate binding site, which in OGC is a symmetrical triplet of arginines. As the substrates malate and oxoglutarate are small, the substrate binding site has only a few asymmetrical adaptations. This Figure is reproduced in colour in the online version of Molecular Membrane Biology.
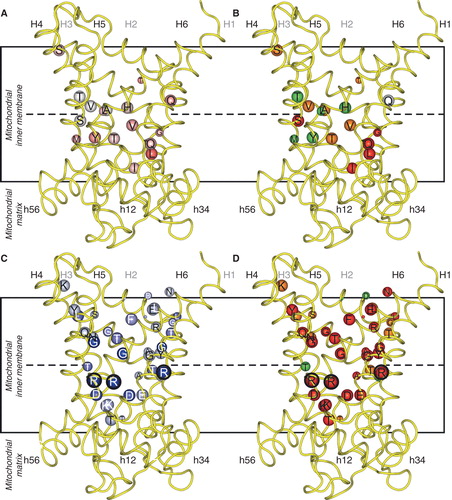
Figure 2. Asymmetry, symmetry and the effects of mutations on transport activity of the yeast phosphate carrier Mir1. Key as in . Where the kinetic parameters were measured, the corresponding initial rates were calculated with the Michaelis-Menten equation at a substrate concentration equal to the km of the wild-type. The black encircled residues are the three contact points of the substrate binding site, which in Mir1 consists of Q86, K179, Q180 and M279, all asymmetric as an adaptation to a small substrate. This Figure is reproduced in colour in the online version of Molecular Membrane Biology.
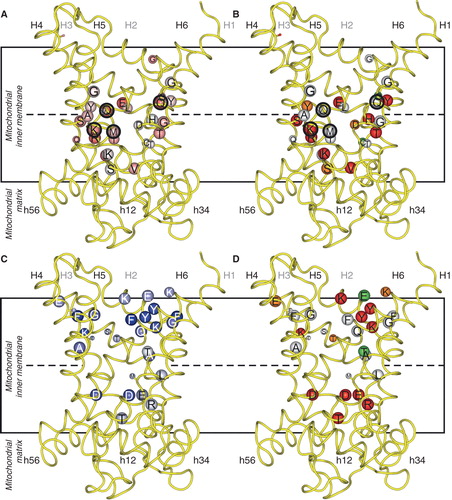
Figure 3. Asymmetry, symmetry and activity of mutants of the yeast citrate carrier Ctp1. Key as in . Where the kinetic parameters were measured, the corresponding initial rates were calculated according to the Michaelis-Menten equation at a substrate concentration equal to the km of the wild-type. The black encircled residues are the contact points of the substrate binding site, which in Ctp1 might consist of the conserved and asymmetric K83 and Q182 together with the symmetric R279 and R181. This Figure is reproduced in colour in the online version of Molecular Membrane Biology.
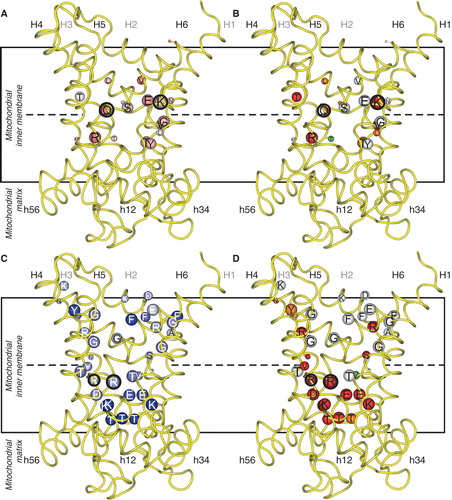
Figure 4. Swapping specificity of the human ornithine carrier by exchanging a single residue in contact point II of the substrate binding site. L-ornithine (magenta) bound in the substrate binding site of ORC1 and ORC2 is shown with the investigated residues (yellow). Results from transport hetero-exchange experiments of radioactive L-ornithine with the wild-type and mutant ORC proteins reconstituted in proteoliposomes, preloaded internally with the various substrates indicated: L-Orn, L-ornithine; L-Arg, L-arginine; L-Lys, L-lysine; L-His, L-histidine; H-Arg, L-homoarginine; D-Orn, D-ornithine; D-Arg, D-arginine and D-Lys, D-lysine (Monné et al. Citation2012). This Figure is reproduced in colour in the online version of Molecular Membrane Biology.
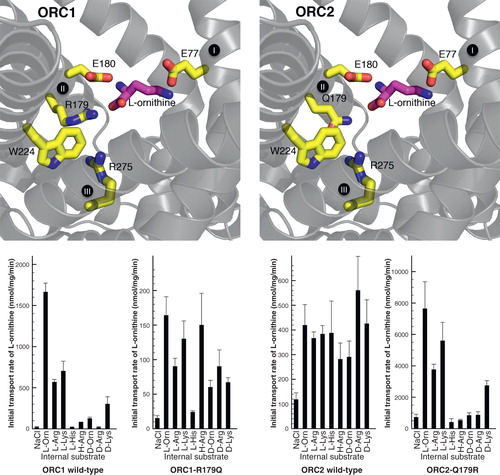
Figure 5. Conserved and symmetrical residues that are critical for function of the mitochondrial oxoglutarate carrier. (A) Residues of the substrate binding site, including the symmetrical and conserved arginine triplet, which are the contact points. (B) Triplets of symmetry-related residues belonging to the GXXXG motif and the PX[DE]XX[RK] motif, which contain a conserved P that is present at the kink and the conserved charge residues of the matrix salt bridge network, which forms in the cytoplasmic state. Underneath are the conserved and symmetrical Q residues, which may interact with the network. (C) Triplets belonging to the aromatic motif [YF]XX[YF] and the cytoplasmic salt bridge network [DE]XX[RK], which are present on the even-numbered α-helices at the cytoplasmic side of the carrier. The charged residues may form a network when the carrier is in the matrix-state. (D) Conserved positively charged [RK] residues, which follow the matrix network and interact with the negatively charged residue of the [ED]G motif. The Y may also be involved in the bonding arrangement, linking the matrix α-helices to the odd-numbered α -helices. The model of OGC, based on the structure of the bovine ADP/ATP carrier, is shown in yellow and the aforementioned residues are shown in red when they are critical to the function, orange if they are important and green when they are not important. This Figure is reproduced in colour in the online version of Molecular Membrane Biology.
![Figure 5. Conserved and symmetrical residues that are critical for function of the mitochondrial oxoglutarate carrier. (A) Residues of the substrate binding site, including the symmetrical and conserved arginine triplet, which are the contact points. (B) Triplets of symmetry-related residues belonging to the GXXXG motif and the PX[DE]XX[RK] motif, which contain a conserved P that is present at the kink and the conserved charge residues of the matrix salt bridge network, which forms in the cytoplasmic state. Underneath are the conserved and symmetrical Q residues, which may interact with the network. (C) Triplets belonging to the aromatic motif [YF]XX[YF] and the cytoplasmic salt bridge network [DE]XX[RK], which are present on the even-numbered α-helices at the cytoplasmic side of the carrier. The charged residues may form a network when the carrier is in the matrix-state. (D) Conserved positively charged [RK] residues, which follow the matrix network and interact with the negatively charged residue of the [ED]G motif. The Y may also be involved in the bonding arrangement, linking the matrix α-helices to the odd-numbered α -helices. The model of OGC, based on the structure of the bovine ADP/ATP carrier, is shown in yellow and the aforementioned residues are shown in red when they are critical to the function, orange if they are important and green when they are not important. This Figure is reproduced in colour in the online version of Molecular Membrane Biology.](/cms/asset/d6f71395-2cdd-48c5-b12f-1f1ae776af65/imbc_a_737936_f0005_b.jpg)