Figures & data
Figure 1. Protein targeting pathways in bacterial and mammalian cells (A) Bacteria engage two targeting pathways for delivering proteins to the SecYEG translocon. The SecA-dependent pathway is used by periplasmic and outer membrane proteins, which contain a cleavable signal sequence. Cytosolic chaperones, including trigger factor and tetrameric SecB keep the nascent polypeptide in a translocation-competent state during its journey to the membrane. The pre-protein is then transferred to SecA, which drives translocation through the SecYEG channel by ATP hydrolysis. Although it is generally assumed that SecA acts post-translationally, some data indicate that SecA can bind to ribosome-nascent chains (RNCs), i.e. that it can also act co-translationally. The SecYEG translocon interacts at least transiently with the SecDFYajC complex, which might support the proton-motif force (pmf)-dependent steps during protein transport. The co-translational SRP-pathway is mainly used for inner membrane proteins and initiated by the ribosome-bound SRP. SRP-RNCs bind to the SecYEG-bound SRP receptor FtsY, RNCs dock onto the SecYEG translocon and the SRP-FtsY complex dissociates in a GTP-dependent manner. During the lateral exit from the SecYEG channel, the nascent membrane protein contactsYidC. YidC is shown to support SecYEG during membrane protein insertion, and it also acts as a SecYEG independent insertase for small or closely spaced membrane proteins. Targeting of membrane proteins to YidC also appears to be SRP-dependent. The translocon associates transiently with several additional proteins, that are required for cleaving the signal sequences of secretory proteins (signal peptidase, SPase), for protein folding (the periplasmic chaperones Skp and PpiD) or for quality control (the membrane-bound protease FtsH). (B) In eukaryotes, the Sec61-mediated insertion and the translocation occurs both co-translationally and post-translationally. During post-translational targeting, fully synthesized pre-proteins are kept in a transport-competent state by members of the Hsp90, Hsp70 and Hsp40 chaperone families. Translocation is mediated by the Sec61 complex in association with Sec62/Sec63 and the chaperone BiP at the lumenal side of the ER membrane. BiP binds to the translocating substrates in the ER lumen and prevents their back-sliding by an ATP-dependent ratcheting mechanism. The eukaryotic SRP pathway delivers both membrane proteins and secretory proteins co-translationally to the Sec61 complex. The eukaryotic SRP receptor consists of two unrelated GTPases, SRα (homologous to FtsY) and SRβ. The Sec61 translocon associates in a substrate-dependent manner with additional proteins that either bind to RNCs (Ramp4) or are suggested to assist membrane protein folding (TRAM, Sec63). BiP is also required for co-translational transport. Like in bacteria, additional proteins are involved in processing and quality control (SPase; TRAP [translocon associated protein], oligosacharyl transferase [OST], the Hsp40-homologue Erj1 or AAA-proteases). This Figure is reproduced in color in the online version of Molecular Membrane Biology.
![Figure 1. Protein targeting pathways in bacterial and mammalian cells (A) Bacteria engage two targeting pathways for delivering proteins to the SecYEG translocon. The SecA-dependent pathway is used by periplasmic and outer membrane proteins, which contain a cleavable signal sequence. Cytosolic chaperones, including trigger factor and tetrameric SecB keep the nascent polypeptide in a translocation-competent state during its journey to the membrane. The pre-protein is then transferred to SecA, which drives translocation through the SecYEG channel by ATP hydrolysis. Although it is generally assumed that SecA acts post-translationally, some data indicate that SecA can bind to ribosome-nascent chains (RNCs), i.e. that it can also act co-translationally. The SecYEG translocon interacts at least transiently with the SecDFYajC complex, which might support the proton-motif force (pmf)-dependent steps during protein transport. The co-translational SRP-pathway is mainly used for inner membrane proteins and initiated by the ribosome-bound SRP. SRP-RNCs bind to the SecYEG-bound SRP receptor FtsY, RNCs dock onto the SecYEG translocon and the SRP-FtsY complex dissociates in a GTP-dependent manner. During the lateral exit from the SecYEG channel, the nascent membrane protein contactsYidC. YidC is shown to support SecYEG during membrane protein insertion, and it also acts as a SecYEG independent insertase for small or closely spaced membrane proteins. Targeting of membrane proteins to YidC also appears to be SRP-dependent. The translocon associates transiently with several additional proteins, that are required for cleaving the signal sequences of secretory proteins (signal peptidase, SPase), for protein folding (the periplasmic chaperones Skp and PpiD) or for quality control (the membrane-bound protease FtsH). (B) In eukaryotes, the Sec61-mediated insertion and the translocation occurs both co-translationally and post-translationally. During post-translational targeting, fully synthesized pre-proteins are kept in a transport-competent state by members of the Hsp90, Hsp70 and Hsp40 chaperone families. Translocation is mediated by the Sec61 complex in association with Sec62/Sec63 and the chaperone BiP at the lumenal side of the ER membrane. BiP binds to the translocating substrates in the ER lumen and prevents their back-sliding by an ATP-dependent ratcheting mechanism. The eukaryotic SRP pathway delivers both membrane proteins and secretory proteins co-translationally to the Sec61 complex. The eukaryotic SRP receptor consists of two unrelated GTPases, SRα (homologous to FtsY) and SRβ. The Sec61 translocon associates in a substrate-dependent manner with additional proteins that either bind to RNCs (Ramp4) or are suggested to assist membrane protein folding (TRAM, Sec63). BiP is also required for co-translational transport. Like in bacteria, additional proteins are involved in processing and quality control (SPase; TRAP [translocon associated protein], oligosacharyl transferase [OST], the Hsp40-homologue Erj1 or AAA-proteases). This Figure is reproduced in color in the online version of Molecular Membrane Biology.](/cms/asset/71c0cfd0-da83-4248-b264-29242716567a/imbc_a_907455_f0001_b.jpg)
Figure 2. The Sec translocon. Schematic representation of the Sec translocon in the closed (A) and the open (B) conformation viewed from the front in the membrane plane (left), as a transverse section through the middle of the pore in the membrane plane (middle) and from the cytoplasmic side (top, right). The open and the closed conformation refer to the lateral gate being closed or open as shown in the front and top representation, respectively. The transverse section and the top view show the pore ring and the plug being displaced for the accommodation of a substrate. (C) Surface representation of the Archaeal SecYEβ translocon in the plane of the membrane (adapted from Van den Berg et al. [Citation2004]; pdb: 1RHZ). The lateral gate helices (TM2b, TM3, TM7 and TM8) of SecY and a short helix (helix 2a), called the plug, are highlighted. The plug is suggested to be involved in sealing the channel. The cytoplasmic loops C4, C5 and C6 of SecY are the major cytoplasmic contact sites for FtsY, SecA and the ribosome. (D) The top view of Sec61YEβ from the cytoplasmic site shows the plug (dark green) sealing the channel and SecE embracing SecY at the back. This Figure is reproduced in color in the online version of Molecular Membrane Biology.
![Figure 2. The Sec translocon. Schematic representation of the Sec translocon in the closed (A) and the open (B) conformation viewed from the front in the membrane plane (left), as a transverse section through the middle of the pore in the membrane plane (middle) and from the cytoplasmic side (top, right). The open and the closed conformation refer to the lateral gate being closed or open as shown in the front and top representation, respectively. The transverse section and the top view show the pore ring and the plug being displaced for the accommodation of a substrate. (C) Surface representation of the Archaeal SecYEβ translocon in the plane of the membrane (adapted from Van den Berg et al. [Citation2004]; pdb: 1RHZ). The lateral gate helices (TM2b, TM3, TM7 and TM8) of SecY and a short helix (helix 2a), called the plug, are highlighted. The plug is suggested to be involved in sealing the channel. The cytoplasmic loops C4, C5 and C6 of SecY are the major cytoplasmic contact sites for FtsY, SecA and the ribosome. (D) The top view of Sec61YEβ from the cytoplasmic site shows the plug (dark green) sealing the channel and SecE embracing SecY at the back. This Figure is reproduced in color in the online version of Molecular Membrane Biology.](/cms/asset/54f60bcf-5cb1-4131-a4c2-373d40d5271a/imbc_a_907455_f0002_b.jpg)
Table 1. Sec-translocon associated proteins and their conservation. Dark grey represents the proteins present in all or most species; light grey represents the proteins found in some species; blank – no known homologue. The paralogues are not indicated.
Figure 3. The ribosomal tunnel exit as a binding platform for targeting factors, chaperones, nascent chain processing enzymes and the translocon. L23, L24 and L29 constitute a universal ribosomal adaptor site. Data are collected from: TF and peptidyl formylase (PDF): (Kramer et al., Citation2002; Bingel-Erlenmeyer et al., Citation2008); SecY: (Frauenfeld et al., Citation2011); SecA: (Huber et al., Citation2011); SRP: (Gu et al., Citation2003; Halic et al., Citation2006; Schaffitzel et al., Citation2006); methionine amino peptidase (MAP): (Sandikci et al., Citation2013); YidC: (Köhler et al., Citation2009; Seitl et al., Citation2013; Welte et al., Citation2012). This Figure is reproduced in color in the online version of Molecular Membrane Biology.
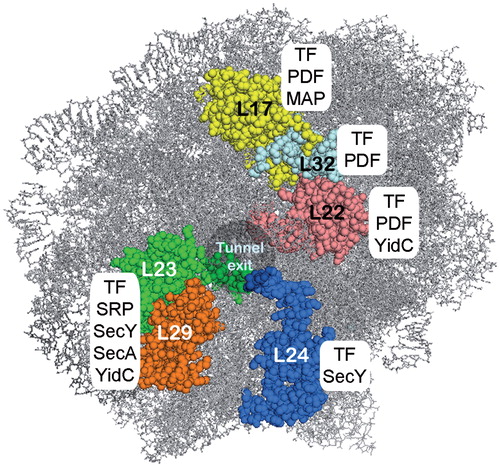
Figure 4. Structure of the signal recognition particle (SRP) and its receptor (SR) (A) Cryo-EM reconstitution of the eukaryotic SRP with the conserved SRP54 subunit, the additional eukaryotic SRP subunits and the 7.5 S RNA (adapted from: (Halic et al., Citation2004); pdb: 1RY1). (B) Crystal structure of the prokaryotic SRP (adapted from (Ataide et al., Citation2011); pdb: 2XXA). The conserved protein subunit Ffh (fifty-four homologue) and the 4.5 S RNA. (C) Crystal structure of the NG-subunit of the bacterial SRP receptor FtsY (adapted from (Ataide et al., Citation2011); pdb: 2XXA) (D) Complex of the prokaryotic SRP and the NG-domain of FtsY (adapted from (Ataide et al., Citation2011); pdb: 2XXA) (E) Crystal structure of the eukaryotic X-domain of SRα in complex with the cytoplasmic domain of SRβ (adapted from Schwartz & Blobel [Citation2003]; pdb: 1NRJ). This Figure is reproduced in color in the online version of Molecular Membrane Biology.
![Figure 4. Structure of the signal recognition particle (SRP) and its receptor (SR) (A) Cryo-EM reconstitution of the eukaryotic SRP with the conserved SRP54 subunit, the additional eukaryotic SRP subunits and the 7.5 S RNA (adapted from: (Halic et al., Citation2004); pdb: 1RY1). (B) Crystal structure of the prokaryotic SRP (adapted from (Ataide et al., Citation2011); pdb: 2XXA). The conserved protein subunit Ffh (fifty-four homologue) and the 4.5 S RNA. (C) Crystal structure of the NG-subunit of the bacterial SRP receptor FtsY (adapted from (Ataide et al., Citation2011); pdb: 2XXA) (D) Complex of the prokaryotic SRP and the NG-domain of FtsY (adapted from (Ataide et al., Citation2011); pdb: 2XXA) (E) Crystal structure of the eukaryotic X-domain of SRα in complex with the cytoplasmic domain of SRβ (adapted from Schwartz & Blobel [Citation2003]; pdb: 1NRJ). This Figure is reproduced in color in the online version of Molecular Membrane Biology.](/cms/asset/9ffad4e3-4739-4391-a9a3-70a24f14a7b1/imbc_a_907455_f0004_b.jpg)
Figure 5. The topology of yeast Sec62, Sec63, Sec71, Sec72 and Sec11. The lumenal J-domain of Sec63 is important for the recruitment of BiP, a chaperone which is essential for Sec61-mediated translocation. The negatively charged C-terminus of Sec63 binds to the N-terminus of Sec62 to collectively support post-translational translocation. Sec71 and Sec72 form the complex with Sec62/Sec63. Sec11 is the catalytic subunit of the yeast signal peptidase complex. This Figure is reproduced in color in the online version of Molecular Membrane Biology.
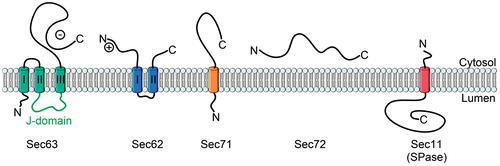
Table 2. The phospholipid composition of the E. coli inner membrane and the ER membrane.
Table 3. Influence of structural lipids of the E. coli inner membrane and the ER membrane on targeting and function of Sec translocon.
Figure 6. Structure of SecA, the motor protein of the post-translational transport in bacteria. (A) Schematic domain organisation of SecA (NBD, Nucleotide binding domains; PBD, peptide-cross-linking domain; HSD, helical scaffold domain; HWD, helical wing domain; CTD, C-terminal domain). (B) Crystal structure of SecA from Thermotoga maritima (adapted from Zimmer et al. (Citation2008); pdb: 3DIN). The colour code is the same as in (A). (C) Crystal structure of SecA in complex with the SecYEG translocon (adapted from Zimmer et al. (Citation2008); pdb: 3DIN). The helices of the lateral gate of SecY are highlighted. This Figure is reproduced in color in the online version of Molecular Membrane Biology.
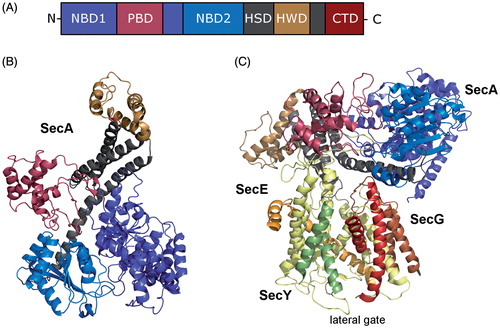
Figure 7. The signal sequence. The signal peptides of the Sec translocon susbtrates in eukaryotes and prokaryotes share a common architecture, with a short, positively charged N-terminal region (N-region), a central, hydrophobic region (H-region) and a polar C-terminal region (C-region). The best conserved part of the signal peptide is the C-region, which can also contain a signal peptidase (SPase) cleavage site. This Figure is reproduced in color in the online version of Molecular Membrane Biology.
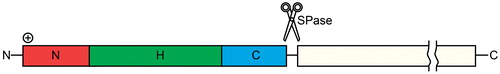
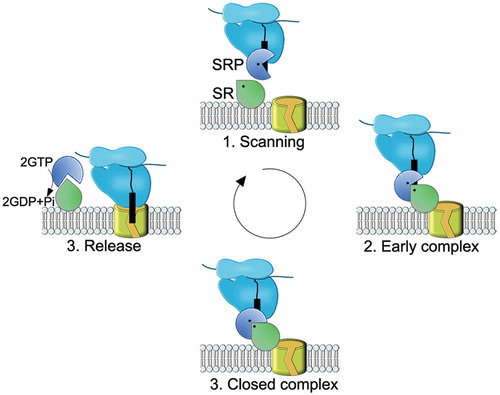
Figure 8. Schematic view of the SRP-SR cycle. The model was adapted from (Akopian et al., Citation2013b). (1) SRP rapidly scans ribosomes and binds stably only to those translating a SRP substrate (2) The presence of the correct substrate increases the affinity of SRP to its receptor (SR), resulting in targeting of the ribosome nascent chain (RNC) to the membrane. This conformation of the SRP-SR complex is termed the early complex. In bacteria, the SRP-SR affinity is further increased if SR is in contact with negatively charged phospholipids. (3) In the presence of the correct substrate, the GTPase domains of SRP and SR align to form a composite GTPase centre (closed complex). To prevent premature dissociation of the SRP-SR complex, GTP hydrolysis is delayed until contacts to the Sec translocon are established. (4) GTP hydrolysis induces the dissociation of SRP-SR complex. SRP-SR GTPases do not require GTP-activating proteins or nucleotide-exchange factors. For SRP, the contact with the ribosome might be sufficient to exchange GDP against GTP. This Figure is reproduced in color in the online version of Molecular Membrane Biology.