Figures & data
Figure 1. Cholesterol (A) and protein (B) content, and cholesterol/protein mass ratio (C) in DRMs obtained by treatment of erythrocytes (RBC) with TX-100, Brij 98, and Brij 58 under different conditions: 4 °C or 37 °C for intact RBC, and 4 °C for cholesterol-depleted RBC (DRMs-MβCD). Cholesterol and protein were quantified in erythrocyte membranes corresponding to 2.5 × 109 RBCs and in their DRMs obtained after centrifugation of the same amount of starting material. **p < 0.001, *p < 0.05 (unpaired Student’s t-test, n = 3–6).
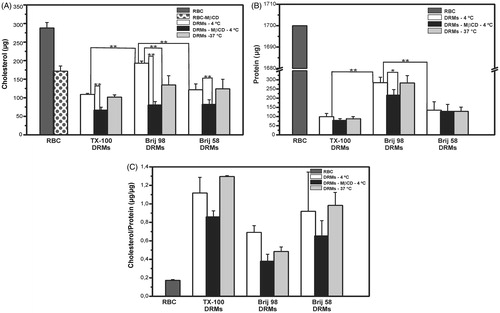
Figure 2. (A) Western blot of the raft markers stomatin and flotillin-2 present in DRM fractions obtained from intact (at 4 °C and 37 °C) and cholesterol-depleted (MβCD, at 4 °C) erythrocytes treated with Brij 98 and Brij 58. (B) Western blot of band 3 present in 10 fractions (from top to bottom, 1–10) obtained at 4 °C and 37 °C. Results are representative of three independent experiments.
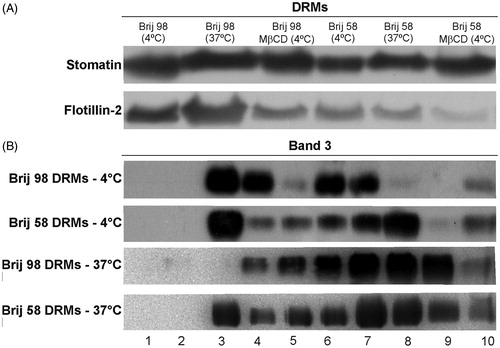
Figure 3. Distribution (mass %) of phospholipid classes (SM – sphingomyelin, PC – phosphatidylcholine, and PE – phosphatidylethanolamine) present in the samples of ghosts, TX-100 DRMs, Brij 98 DRMs, and Brij 58 DRMs analyzed by HPTLC and quantified by densitometry. DRMs were obtained from erythrocytes treated with detergents at 4 °C.
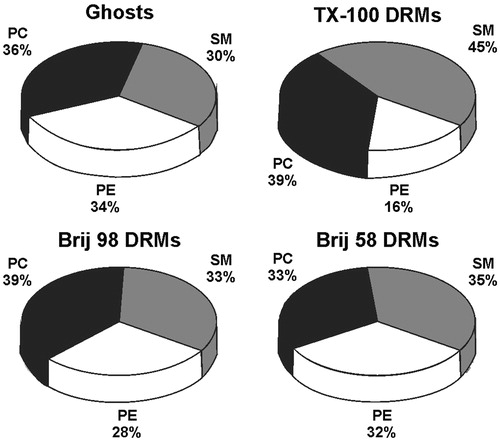
Figure 4. Distributions of fatty acids in the phosphatidylcholine (A), phosphatidylethanolamine (B), and sphingomyelin (C) fractions extracted by HPTLC from ghost membranes or DRMs, as shown in . The saturated/unsaturated fatty acids ratios in the PC, PE, and SM fractions present in ghost membranes and DRMs is given in (D). Palmitic acid (16:0), stearic acid (18:0), oleic acid (18:1), linoleic acid (18:2), arachidonic acid (20:4), behenic acid (22:0), lignoceric acid (24:0), nervonic acid (24:1) (only detected in SM fractions). *p < 0.001 and **p < 0.05: a ghosts vs. all DRMs, b ghosts vs. TX-100 and Brij 58 DRMs, c ghosts vs. Brij 98 DRMs, d Brij 98 DRMs vs. TX-100 DRMs, e Brij 58 DRMs vs. TX-100 DRMs (unpaired Student’s t-test, n = 3). DRMs were obtained from erythrocytes treated with detergents at 4 °C.
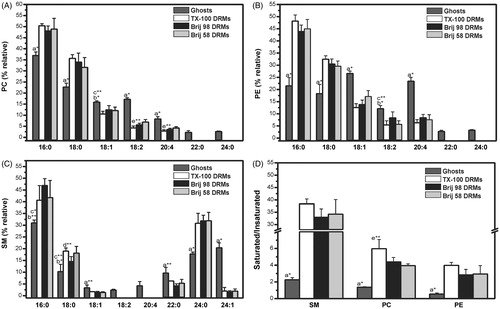
Figure 5. EPR spectra of 5-SASL in intact erythrocytes (RBC), TX-100, Brij 98, and Brij 58 DRMs (A). Order parameter (S) values obtained from EPR spectra of 5- and 16-SASL (B and C, respectively) in intact (RBC) and cholesterol-depleted (MβCD) erythrocyte cells and their respective DRMs prepared at 4 °C or 37 °C with TX-100, Brij 98, and Brij 58. All EPR spectra were recorded at 25 °C. **p < 0.001, *p < 0.05 (unpaired Student’s t-test, n = 3–6).
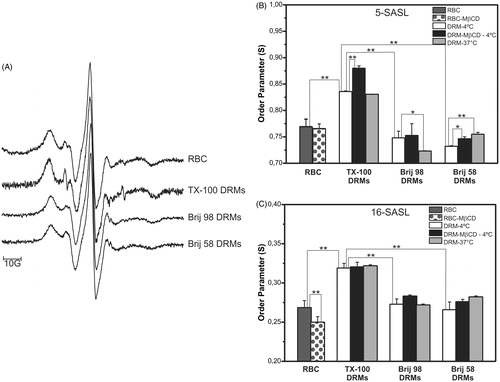
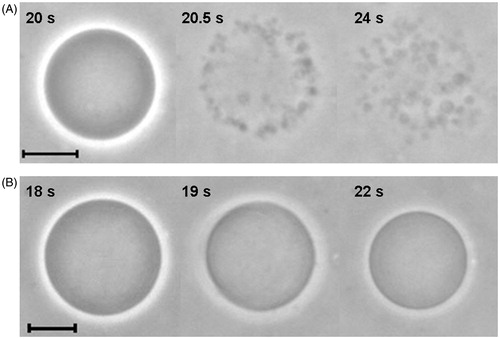
Figure 6. Sequence of images of erythro-GUVs in the presence of (A) 0.1 mM Brij 98 and (B) 0.5 mM TX-100. The time shown in each snapshot is relative to the moment when the erythro-GUVs were added to the detergent suspension. At least 20 GUVs were followed in the presence of Brij 98 and 100 GUVs in the presence of TX-100. The scale bars represent 10 μm.