Figures & data
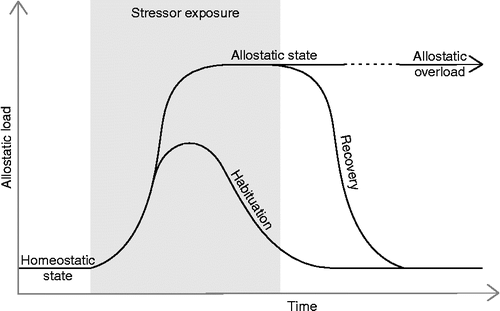
Figure 1. A hypothetical model of retrograde eCB signaling at a glutamatergic synapse. (a) Glutamate (Glu) is released at moderate levels activating postsynaptic ionotropic glutamate receptors leading to an excitatory postsynaptic potential (EPSP). (b) Increased or prolonged glutamate release additionally activates G-protein coupled metabotropic glutamate receptors (mGluR). A secondary messenger cascade leads to the synthesis of eCB. (c) Newly synthesized eCBs travel back across the synapse to activate G-protein coupled CB1 receptors. (d) CB1 receptor activation inhibits further neurotransmitter release by blocking calcium Ca2+ influx into the presynaptic cell among other actions. Glutamate release is downregulated and the EPSP is attenuated (figure modified from Moreira and Wotjak Citation2010).
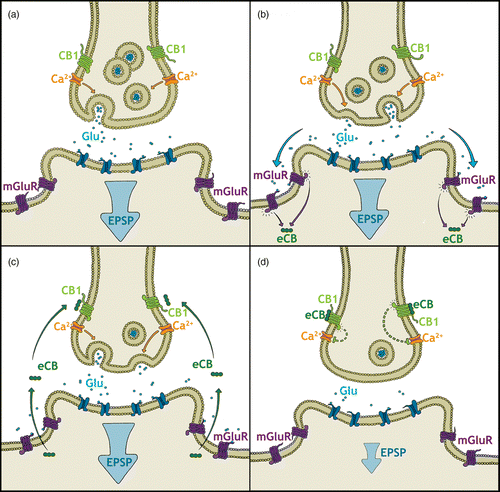
Figure 2. The HPA-axis and its glucocorticoid feedback mechanisms including exemplary eCB-mediated negative feedback in the PVN. (a) Neuronal input into the HPA-axis from psychological stressors originates from cortical-based cognitive and decision-making brain areas, whereas input from physical stressors is from lower hindbrain regions (Herman et al. Citation2003). These inputs culminate in the activation of neurosecretory cells in the PVN and the release of CRH. In the anterior pituitary, CRH stimulates the release of ACTH into circulation where it activates glucocorticoid production in the adrenal cortex. Glucocorticoids travel to the periphery to have widespread physiological effects as well as providing negative feedback to the HPA-axis via several brain areas, including the PVN, PFC, and hippocampus (HPC; de Kloet et al. Citation2005; Pecoraro et al. Citation2006). (b) Glucocorticoid fast feedback in the PVN stimulates membrane-associated GRs, which in turn leads to the synthesis of (eCBs; Di et al. Citation2003; Tasker et al. Citation2006). (c) eCBs activate presynaptically located CB1 receptors to inhibit further neurotransmitter release. Glutamatergic activation of EPSPs in the postsynaptic neurosecretory cell is reduced, subsequently leading to decreased CRH release and downregulation of HPA-axis activity (Di et al. Citation2003; Tasker et al. Citation2006).
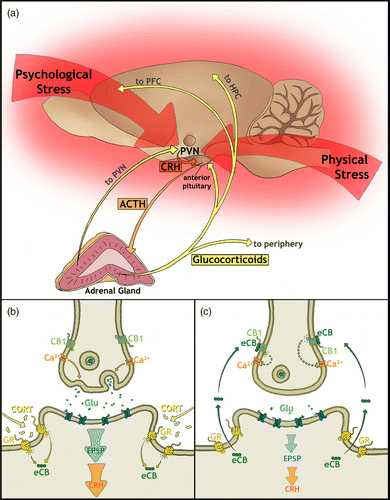
Figure 3. A schematic representation of the relationship between homeostatic and allostatic states. Allostatic load is the relative cost of the physiological responses to stress, such as HPA-axis activation (McEwen Citation1998; McEwen and Wingfield 2003). Prior to stressor exposure, homeostatic functioning is at a low allostatic load and eCB signaling helps maintain homeostasis by counteracting HPA-axis activation (Patel et al. Citation2004; Steiner and Wotjak Citation2008; Hill et al. Citation2009b; Ginsberg et al. Citation2010). During some stress protocols habituation-like processes may occur, which effectively reduce allostatic load and help reinstate homeostatic functioning. The eCB system evidently plays a key role in stress-induced habituation-like processes (Patel et al. Citation2005; Patel and Hillard Citation2008; Hill et al. Citation2010b). In cases where habituation does not occur, a relatively high incurred allostatic load may result in the development of an allostatic state. Several studies have investigated the role of eCB signaling in stress protocols in which habituation does not occur (Martin et al. Citation2002; Hill and Gorzalka Citation2004; Hill et al. Citation2005; Bortolato et al. 2007). Upon stressor termination, allostatic states may prevail leading to allostatic overload and conditions of chronic stress such as burnout, anxiety, and depression in humans. Evidence indicates that upregulation of eCB signaling may help prevent depression-like symptoms in animal models (Hill and Gorzalka Citation2005; Bambico and Gobbi Citation2008; Gorzalka et al. Citation2008; Hill et al. Citation2009a), but its role in the stress-induced development of these states is unclear. Recovery processes from allostatic states may occur to reduce allostatic load and reinstate homeostatic functioning. It has yet to be investigated what role eCB signaling may play in these recovery processes.