Figures & data
Table 1. Neonicotinoid insecticides examined in guideline developmental neurotoxicity studies and structural comparison to nicotineTable Footnotea.
Table 2. Comparative acute toxicity and nicotinic receptor binding of substrates in insects and mammals.
Table 3. Inclusion criteria used to evaluate publications in this review.
Table 4. Criteria used to evaluate the strength and relevance of studies included in this review.
Table 5. Results of published studies that examined neonicotinoid insecticides for developmental neurotoxicity.
Table 6. Principal elements of EPA and OECD guideline developmental neurotoxicity studyTable Footnotea.
Table 7. Laboratory-specific test procedures for guideline developmental neurotoxicity studies.
Table 8. Statistical analyses used in guideline developmental neurotoxicity studies.
Table 9. Summary of results for guideline developmental neurotoxicity studiesTable Footnotea.
Figure 1. Motor activity for PND 21 (top) and PND 60 (bottom) F1 male rats (n = 10/sex/dose level) exposed to acetamiprid via gavage administration to the dam (GD 6 to LD 21). There were no statistically significant results based ANOVA for each trial for males or females, separately, or when data were combined for males and females and analyzed using repeated measures ANOVA and Dunnett’s (see text).
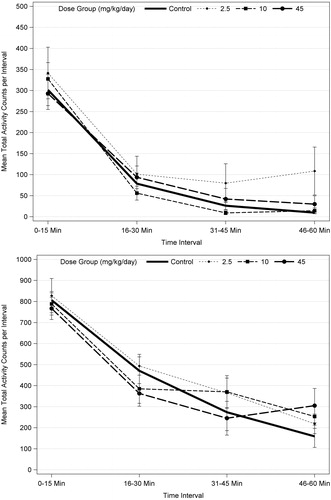
Figure 2. Mean (±SEM) number of errors per trial on Biel water maze (8 unit T-test) in F1 male offspring (10/sex) on PND 22 (top) and PND 62 (bottom), following developmental exposures to acetamiprid. Both males and females were tested for 7 d with four swim trials on a straight channel on the first day (no effect; data not shown); 2 trials/day on Path A for 2 d (A1–A4), 2 trials/d on path B (reverse of path A; B5–B10) for 3 d; and two trials on path A (A11–A12) on the last day (probe test). A different set of animals was tested on PND 22 and PND 62. The number of errors (deviation from correct channel with all four paws) and the time required for the animal to escape (data not shown) were recorded. EPA (2008) identified possible trend in errors only in high-dose males at PND 22, but also noted there were no effects on latency. There was no statistical significance at either age when males and females were analyzed separately (ANOVA and Dunnett’s for each trial; α = 0.05). When males and females were combined (n = 20 litters/dose group) and analyzed using repeated measures ANOVA with trials as the repeated measures, there was a significant effect of trials (p < 0.018 or p < 0.001) but no significant dose effects or dose interactions with sex or trial for each age (α = 0.05).
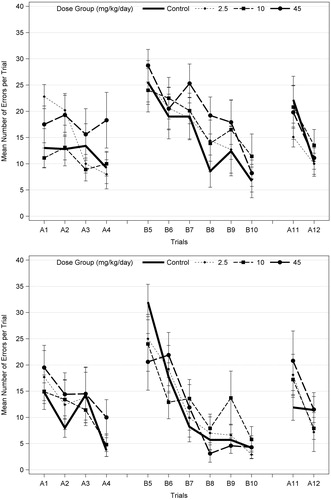
Table 10. Caudate-putamen width (mm) in F1 rats treated with imidaclopridTable Footnotea.
Table 11. Trials-to-criterion and latency (sec) for passive avoidance in F1 Sprague–Dawley rats treated with thiaclopridTable Footnotea.
Table 12. Brain measurements (μm) in F1 rats treated with thiaclopridTable Footnotea.
Table 13. Number of movements (counts) in F1 animals treated with clothianidinTable Footnotea.
Table 14. IRAC classification of chemicals interacting with the nAChRTable Footnotea.
