Figures & data
Figure 1. Unresolved questions in the early evolution of eukaryotes. (A) How many domains of life are there? The traditional view of the tree of life places all three of the major domains, i.e. bacteria, archaea and eukaryota as monophyletic (top tree). This implies that the eukaryotes branched from the Archaea as a separate and independent lineage, with a stepwise topology, i.e. bacteria emerged first, from which archaea arose and then finally the eukaryota. An alternate hypothesis, however, suggests that the eukaryotes are essentially a branch within the archaea, and that archaea and eukaryota are, therefore, monophyletic (lower tree), allowing for coevolution of an archaeal/eukaryote precursor prior to speciation. Attempts to reconstruct the topology of the achaeal/eukaryota differentiation have so far been inconclusive, with both models receiving support, although this support is far from unequivocal (Gribaldo et al., Citation2010). OoL; origin of life, FECA; first eukaryotic common ancestor. (B) When did the mitochondrial symbiont arrive? Many proposals for the origin of the first eukaryotic cell have been offered. Of the models that have garnered support, two simple common schema can be extracted, and a third more complex possibility. Left; A fusion event occurred between an archaea and an α-proteobacterium (the source of the mitochondrial genome and functions). Central; Significant development of endogenously-derived membranous and other structures by the Archaeal ancestor arose prior to endosymbiosis of the α-proteobacterium. The latter mechanism may have involved more complex fusion events, for example including methanogens or an endosymbiotic origin for the nucleus (discussed in Embley & Martin, Citation2006), while the metabolic capabilities of ancestral cells are essentially ignored. Right; A third possibility is that the mitochondrion arose comparatively late, after much of the complexity of the protoeukaryote had evolved, and following fusion between a bacterium (khaki lozenge) and an archaeon (right scheme). While multiple endosymbiont events are considered by many as highly unlikely, the point at which the mitochondrion came on board, as well as when a true nucleus arose remain controversial and unresolved. However, most models agree that the LECA possessed mitochondria, substantial internal differentiation and a well-defined nucleus. Probing beyond LECA is critical for understanding these earliest events. Gray lozenge; Archaeal ancestor, purple lozenge; α-proteobacteria (the mitochondrion is drawn in purple in the LECA), blue lozenge; protonuclear endosymbiont (the nucleus is drawn in blue in the LECA). (C) Eukaryotic tree of life with examples of sequenced organisms from currently recognized supergroups. The curved dotted-line indicates the separation of lineages included in the unikonts and the bikonts. SAR + CCTH: stramenopiles (heterokonts), alveolates, and Rhizaria plus cryptomonads, centrohelids, telonemids and haptophytes (see color version of this figure at www.informahealthcare.com/bmg).
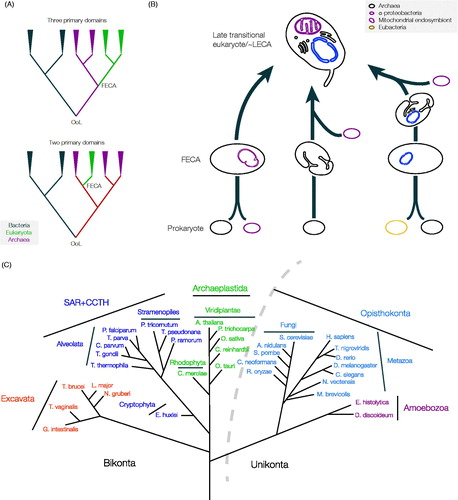
Figure 2. Possible scenarios for the FECA to LECA transition. The top schema depicts the periods of prokaryotic (blue) and eukaryotic (red) evolution, separated by a transition period, which is expanded for clarity. Relative distances on the x-axis are arbitrary, and note that the earliest times shown are post origin of life. It is assumed that prokaryotic and eukaryotic evolution resulted in an increase in cellular complexity, denoted by the blue and red triangles, respectively. The possibility that eukaryotes evolved before prokaryotes is not discussed. It is unknown if FECA (red arrow head) and the origin of the nucleus, acquisition of the mitochondrion or internal compartments (green, purple and yellow arrow heads) are coincident, or near coincident events, despite the possibility that the nucleus evolved from simpler progenitor structures. It is also unclear if the origin of the nucleus is the earliest event in the transition period; for example it is possible to envisage other scenarios, i.e. where endosymbiosis of the mitochondrion ancestor came before acquiring the nucleus, and that this event, rather than formation of a nucleus (either by gradual steps or by fusion), was the initial event that produced FECA. During the transition period the LECA ancestor’s trajectory is shown as a solid line with a sharp increase in complexity, but other possibilities cannot be discounted (faint line; multiple transitions). Other trajectories that could be envisaged are not shown for purposes of clarity only. It is assumed that the LECA ancestor was just one of many lineages that arose from the single eukaryogenesis event, but that it came to dominate or integrate with other lineages. Examples of extant taxa and their approximate complexity given at right in the top panel, simply to illustrate that some extant eukaryotes are likely less complex than LECA, and that there is overlap in complexity between prokaryotic and eukaryotic organisms; note that complexity itself is a difficult term, and here is taken as a composite of genomic and cellular functional complexity/differentiation. The lower schemas illustrate two of the major hypotheses, the syntrophic and phagotrophic models (left and centre, respectively), that suggest that the mitochondrion (purple arrowhead, MT) was the first event or that evolution of the nucleus (green arrowhead, N) and more complex intracellular structures (yellow arrowhead, internal membranes, IM) occurred prior to phagocytosis of the mitochondrial ancestor. A third complex path, that incorporates additional evolvable systems like a sophisticated cytoskeleton (blue arrowhead, other), leading to a double transition after the mitochondrion/nucleus is also shown. Excellent arguments in favour of the first two models have been advanced, but due to the contingent nature of eukaryogenesis, a great many possibilities remain (see color version of this figure at www.informahealthcare.com/bmg).
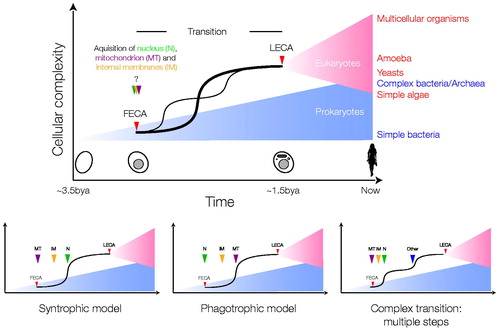
Figure 3. A generalized model for LECA with emphasis on the major systems proposed as present and discussed here. It is now clear that the LECA was both a flagellate and capable of movement by actin-based pseudopodia and possessed a sophisticated cytoskeleton, including large families of kinesin and dynein motors (not shown for clarity). It possessed a complex and likely very flexible, metabolism and a fully functional mitochondrion. Endomembrane compartments would have been essentially indistinguishable from modern cells, and included the endoplasmic reticulum, the Golgi complex, endosomes, autophagosomes and others (many not shown for clarity). The LECA was also capable of both conventional endocytosis and phagocytosis. The nucleus was fully differentiated with nuclear pore complexes and a sophisticated system for organization and regulation of chromatin. A high energy burden is clearly implied by this architecture and required to construct and maintain these compartments and systems plus a differentiated cytoskeleton to coordinate location and function. Heterochromatin in some form could also support life-cycle and/or environmental cue-dependent coordinate gene expression. LECA also supported meiosis. Systems are exploded with examples of the complex aspects associated with a reconstructed LECA (see color version of this figure at www.informahealthcare.com/bmg).
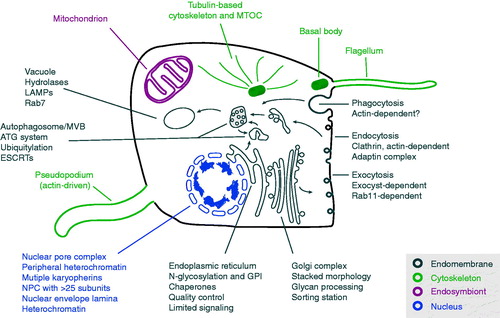
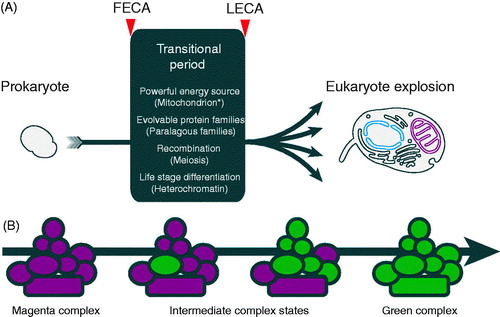
Figure 4. FECA to LECA transitions and flexible evolution of paralog complexes. (A) Transition of prokaryotes to eukaryotes during the period between FECA and LECA, and which incorporated a number of highly significant features. It is unresolved as to which of these occurred first, and only in the case of the acquisition of the mitochondrion, is it well agreed that this a singular event. It was only once all of these features were in place that the LECA was poised for the explosive differentiation of the eukaryotic lineage. (B) Flexibility in protein complex evolution. Rapid success for the LECA ancestors may have required evolvability within protein complexes, resulting in the large number of paralogs in modern eukaryotes. Complexes built from paralogs have an intrinsic evolutionary advantage in allowing new paralogs rapid access to functionality; if a substantial proportion of a complex is built using paralogs this potential is increased. For example, if a single subunit of the magenta complex is replaced by a paralog (green), but which initially is identical to the original paralog, this provides the opportunity for one of the paralogs to drift by acquisition of mutations. This process can then either relax sequence restraints on other subunits or even select for changes that facilitate neofunctionalisation. These other subunits can also be replaced by new paralogs, which is made more probable by the original paralogous expansion. The process is completed by the achievement of a fully green complex, but there are many examples of subunits being shared between complexes with bona fide distinct functions; this may reflect either the achievement of some maximal functionality or reflect an incomplete evolutionary change. See Dacks & Field (Citation2007) for a more detailed discussion of this concept as applied to the trafficking system (see color version of this figure at www.informahealthcare.com/bmg).