Figures & data
Figure 1. Immunofluorescence double staining of transglutaminase II and tenascin-C in non-neoplastic lymphoid tissues. Transglutaminase II (rhodamine, red arrows) and tenascin-C (FITC, green arrows) were mainly expressed in the marginal zone and T-cell area in a reactive lymph node (A) and tonsil (B). Double positive cells of transglutaminase II and tenascin-C were also detected in the same areas (merged, yellow arrows).
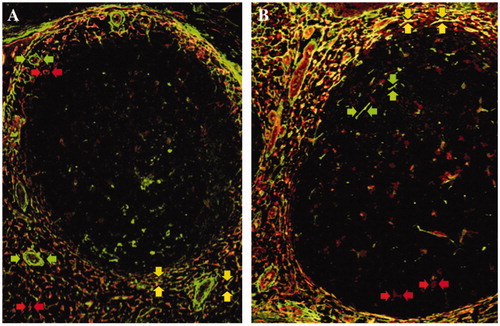
Figure 2. The expression of collagen modifying enzymes in non-neoplastic lymphoid tissues. The expression of prolyl 4-hydroxylase 1 (A), lysyl hydroxylase 3 (B), and protein disulfide isomerase (C) was more frequently observed in the marginal zone (MaZ) and germinal center (GC) than in the mantle zone (MnZ) or interfollicular area (IF). A, B, and C are serial sections of the tonsil. Transglutaminase II (D and F; rhodamine, red arrows) was predominantly expressed in the marginal zone and T-cell area, as was lysyl hydroxylase 3 (E and F; FITC, green arrows) as well as in the germinal center in a lymph node. Double positive cells of transglutaminase II and lysyl hydroxylase 3 were localized in the marginal zone and T-cell area (F; merged, yellow arrows).
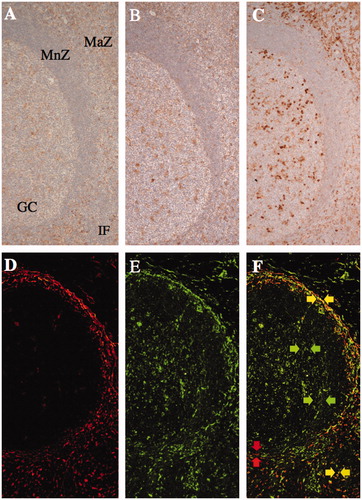
Table I. The staining index of transglutaminase II, tenascin-C, and collagen modifying enzymes on fibroblastic reticular cells and follicular dendritic cells in non-neoplastic lymph nodes.
Table II. Comparison of the staining index of collagen modifying enzymes and the frequency of transglutaminase II+ fibroblastic reticular cells and CD35+ follicular dendritic cells between malignant lymphomas and their respective postulated normal counterparts.
Figure 3. Comparison of three selected collagen modifying enzymes on transglutaminase II+ fibroblastic reticular cells or CD35+ follicular dendritic cells in between malignant lymphomas and postulated normal counterparts. Prolyl 4-hydroxylase 1 (P4H1) was expressed in the germinal center (GC) and marginal zone (MaZ) (A; FITC, green colored) and transglutaminase II+ fibroblastic reticular cells (TGII+ FRCs) were localized in the MaZ (A; rhodamine, red colored) in the tonsil. There were a few of P4H1+TGII+ FRCs in MaZ (A; merged, yellow colored and arrows). In nodular sclerosis classical Hodgkin lymphoma, P4H1+TGII+ FRCs were proliferating (B; merged, yellow colored) at the background of Hodgkin–Reed–Sternberg cells (data not shown). Lysyl hydroxylase 3 (LH3) was expressed in the GC (C; FITC, green colored) and CD35+ follicular dendritic cells (CD35+ FDCs) were localized in the GC (C; rhodamine, red colored) in the tonsil. There were a lot of LH3+CD35+ FDCs in GC (C; merged, yellow colored). In follicular lymphoma, there were a few of LH3+CD35+ FDCs in the neoplastic follicle (D; merged, yellow colored and arrows). Protein disulfide isomerase (PDI) was expressed in the GC (E; rhodamine, red colored) and CD35+ FDCs were localized in the GC (E; FITC, green colored) in the tonsil. PDI+CD35+ FDCs were scattered in GC (E; merged, yellow colored and arrows). In follicular lymphoma, no PDI+CD35+ FDCs was existed in the neoplastic follicle (E; FITC, green colored).
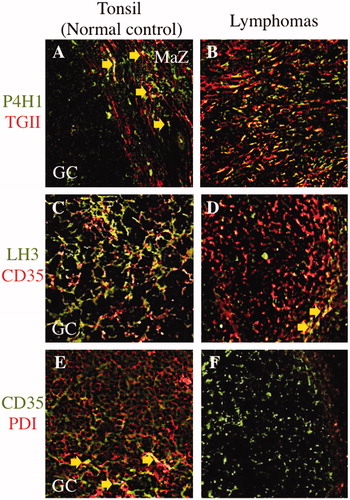
Figure 4. Immunofluorescence double staining of transglutaminase II and CD35 in malignant lymphomas and a non-neoplastic lymphoid follicle. In normal tonsil, transglutaminase II+ fibroblastic reticular cells (TGII+ FRCs) were mainly localized in the marginal zone and T-cell area (A; rhodamine, red colored), and CD35+ follicular dendritic cells (CD35+ FDCs) were localized in the germinal center (A; FITC, green colored). In follicular lymphoma, TGII+ FRCs were scattered in a neoplastic follicle (B; rhodamine, red colored) with the background of densely packed CD35+ FDCs (B; FITC, green colored). In angioimmunoblastic T-cell lymphoma, TGII+ FRCs (C; rhodamine, red colored) were intermingled with CD35+ FDCs meshwork (C; FITC, green colored). In nodular sclerosis classical Hodgkin lymphoma, TGII + FRCs were proliferating (D; rhodamine, red colored) around an atrophic follicle of CD35 + FDCs (D; FITC, green colored). Erythrocytes existed intravascular areas (B and C; merged, yellow arrows).
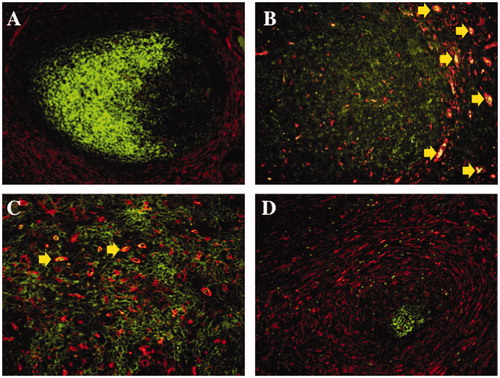
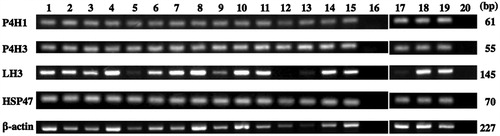
Figure 5. Reverse transcription polymerase chain reaction of collagen modifying enzymes. Positive controls for mRNAs of prolyl 4-hydroxylase 1 (P4H1), prolyl 4-hydroxylase 3 (P4H3), lysyl hydroxylase 3 (LH3), and heat shock protein 47 (HSP47) were shown in lanes 1 and 17. The mRNAs of P4H1, P4H3 and HSP47 were expressed in non-neoplastic tonsils and lymph nodes (lanes 2 and 3, respectively) and lymphomas (lane 4, small lymphocytic lymphoma; lane 5, nodal marginal zone lymphoma; lane 6, extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue; lane 7, mantle cell lymphoma; lanes 8 to 10, follicular lymphoma grades 1, 2 and 3, respectively; lane 11, peripheral T-cell lymphoma; lane 12, enteropathy-associated T-cell lymphoma; lane 13, angioimmunoblastic T-cell lymphoma; lane 14, nodular sclerosis classical Hodgkin lymphoma; lane 15, mixed cellularity classical Hodgkin lymphoma; lane 18, diffuse large B-cell lymphoma not other specified, germinal center B-cell-like subgroup; lane 19, diffuse large B-cell lymphoma not other specified, non-germinal center B-cell-like subgroup), while LH3 mRNA was expressed in most cases, except for enteropathy-associated T-cell lymphoma. Lanes 16 and 20, negative control.