Figures & data
Table I. OCR of pharmacological ascorbate in DMEM growth media.*
Figure 1. Ascorbate increases intracellular concentration of H2O2. A. MIA PaCa-2 cells were treated with L-ascorbate 2-phosphate ([A2P] = 100 μM) for 24 h and ascorbate (2 mM) for 1 h and clonogenic survival was determined. A combination of A2P (100 μM) and ascorbate (2 mM) did show a significant decrease in survival compared to controls. (*P < 0.01 vs. control, means ± SEM, n = 3). However, this treatment did not show significant decreases in clonogenic survival when compared to ascorbate-treated cells, suggesting that increased intracellular ascorbate levels were not responsible for ascorbate-induced cytotoxicity under our experimental conditions. B. MIA PaCa-2 cells were treated with 20 mM ascorbate in the presence of 20 mM aminotriazole (AT) and the concentration of intracellular H2O2 was determined using aminotriazole-mediated inactivation of endogenous catalase. Cells treated with ascorbate showed a significant 66 % increase in intracellular H2O2 when compared to cells that were treated with AT only, *P < 0.05 vs. AT, means ± SEM, n = 4.
![Figure 1. Ascorbate increases intracellular concentration of H2O2. A. MIA PaCa-2 cells were treated with L-ascorbate 2-phosphate ([A2P] = 100 μM) for 24 h and ascorbate (2 mM) for 1 h and clonogenic survival was determined. A combination of A2P (100 μM) and ascorbate (2 mM) did show a significant decrease in survival compared to controls. (*P < 0.01 vs. control, means ± SEM, n = 3). However, this treatment did not show significant decreases in clonogenic survival when compared to ascorbate-treated cells, suggesting that increased intracellular ascorbate levels were not responsible for ascorbate-induced cytotoxicity under our experimental conditions. B. MIA PaCa-2 cells were treated with 20 mM ascorbate in the presence of 20 mM aminotriazole (AT) and the concentration of intracellular H2O2 was determined using aminotriazole-mediated inactivation of endogenous catalase. Cells treated with ascorbate showed a significant 66 % increase in intracellular H2O2 when compared to cells that were treated with AT only, *P < 0.05 vs. AT, means ± SEM, n = 4.](/cms/asset/095b0aa3-ad9c-4a25-a9c8-8919a515530b/ifra_a_755263_f0001_b.gif)
Figure 2. Ascorbate treatment induces oxidative stress. A. MIA PaCa-2 cells were treated with ascorbate (2 mM) for 0, 2, 6, and 24 h and intracellular GSH levels were measured. Ascorbate (2 mM) treatment decreases intracellular GSH. *P < 0.05 vs. the 0 time point, means ± SEM, n = 3. B. Ascorbate (2 mM) increases (more positive) the intracellular half-cell reduction potential (Ehc) of the intracellular GSSG/2GSH couple (intracellular redox buffer) of MIA PaCa-2 cells, a clear indication of increased intracellular oxidations. *P < 0.05 vs. the 0 time point, means ± SEM, n = 3. C. Intracellular GSH decreases with increasing concentrations of extracellular ascorbate (2 – 20 mM for 1 hour). *P < 0.05 vs. the 0 mM dose, means ± SEM, n = 3. D. The half-cell reduction potential of MIA PaCa-2 cells increases in a dose-dependent manner demonstrating ascorbate-induced oxidation. *P < 0.05 vs. the 0 mM dose, means ± SEM, n = 3.
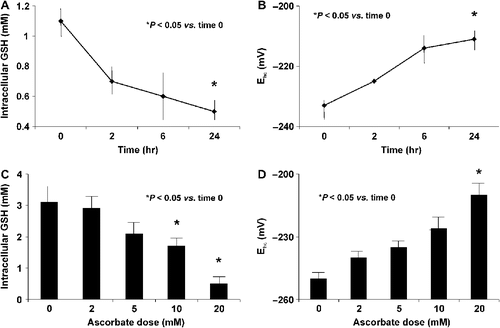
Figure 3. BCNU enhances ascorbate-induced cytotoxicity through the inhibition of glutathione disulfide reductase. A. BCNU decreases the activity of glutathione disulfide reductase in a dose-dependent manner. *P < 0.05 vs. the BCNU 0 μM dose, means ± SEM, n = 3. B. MIA PaCa-2 cells treated with BCNU (25 μM) showed decreasing activity of GR over time. *P < 0.05 vs. the BCNU 0 μM dose, means ± SEM, n = 3. C. MIA PaCa-2 cells were treated with BCNU (25 μM, 2 h) and ascorbate (2 mM, 1 h) and clonogenic survival was determined. Ascorbate caused a decrease in clonogenic survival, *P < 0.001 vs. control, mean ± SEM, n = 3. D. AsPC-1 cells were treated with BCNU (25 μM) and ascorbate (2 mM) and clonogenic survival was determined. Ascorbate caused a decrease in clonogenic survival, *P < 0.001 vs. control, mean ± SEM, n = 3. E. MIA PaCa-2 cells were treated with BCNU (25 μM, 2 h) and ascorbate (2 mM, 1 h) and cell viability with a MTT assay was determined. Ascorbate caused a decrease in cell viability, *P < 0.01 vs. control, mean ± SEM, n = 4. Treatment with BCNU alone showed no significant effect on cytotoxicity, but a combination of BCNU and ascorbate enhanced ascorbate-induced cytotoxicity, **P < 0.05 vs. ascorbate, means ± SEM, n = 4. Similar trends were seen with AsPC-1 cells treated with ascorbate ± BCNU (data not shown).
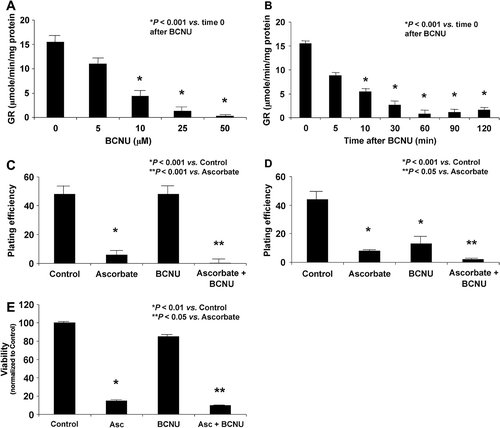
Figure 4. siRNA to GR enhances ascorbate-induced cytotoxicity. A. Cells were transfected with a siRNA against GR. The Western blot shows that GR protein was significantly decreased in siGR-transfected cells in comparison to controls. B. GR activity was decreased in cells treated with siGR compared to controls or cells treated with siNEG. C. MIA PaCa-2 cells were transfected with a siRNA against GR and treated with ascorbate (2 mM for 1 h) and clonogenic survival was determined. Treatment with siGR had little effect on clonogenic survival while cells treated with ascorbate showed a significant decrease in clonogenic survival, *P < 0.001 vs. control, means ± SEM, n = 3. Additionally, treatment with a combination of siGR and ascorbate enhanced ascorbate-induced cytotoxicity (**P < 0.001 vs. ascorbate, means ± SEM, n = 3).
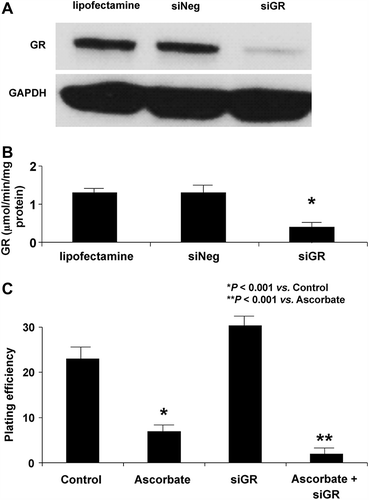
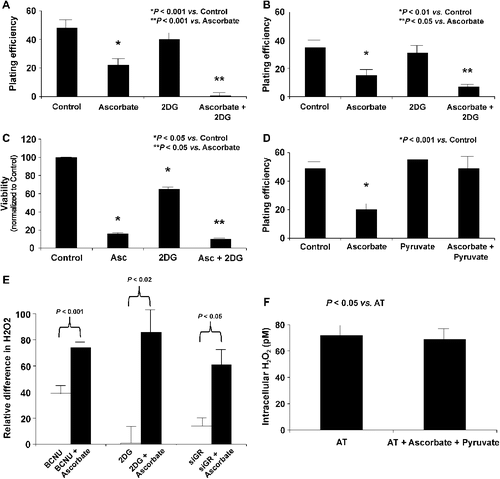
Figure 5. Inhibitors of H2O2 removal enhance ascorbate-induced cytotoxicity and increase intracellular concentrations of H2O2. A. Mia PaCa-2 cells were treated with 2DG (25 mM) and ascorbate (2 mM) for 1 h and clonogenic survival was determined. Ascorbate treatment caused a decrease in clonogenic survival (*P < 0.001 vs. control, means ± SEM, n = 12). Treatment with 2DG (25 mM) did not affect clonogenic survival but the combination of 2DG (25 mM) and ascorbate showed a significant enhancement in ascorbate-induced cytotoxicity, **P < 0.001 vs. ascorbate, means ± SEM, n = 6. B. AsPC-1 cells were treated with 2DG (25 mM) and ascorbate (2 mM) for 1 h and clonogenic survival was determined. Ascorbate treatment caused a decrease in clonogenic survival (*P < 0.01 vs. control, means ± SEM, n = 3). Treatment with 2DG (25 mM) did not affect clonogenic survival but the combination of 2DG (25 mM) and ascorbate showed a significant enhancement in ascorbate-induced cytotoxicity, **P < 0.05 vs. ascorbate, means ± SEM, n = 3. C. MTT assay demonstrated significant decreases in cell viability in MIA PaCa-2 and AsPC-1 cells treated with the combination of ascorbate and 2DG. Ascorbate significantly decreased viability while the combination of ascorbate and 2DG further decreased viability (n = 4, *P < 0.05 vs. control, **P < 0.05 vs. ascorbate). Similar trends were seen with AsPC-1 cells treated with ascorbate ± 2DG (data not shown). D. Cells were treated with pyruvate (5 mM) and ascorbate (2 mM) for 1 h and clonogenic survival determined. Cells treated with ascorbate showed a significant decrease in clonogenic survival (*P < 0.001 vs. control, means ± SEM, n = 3). Pyruvate (5 mM), a scavenger of H2O2 was able to reverse the toxicity of ascorbate. Pyruvate alone (5 mM) did not affect clonogenic survival. E. MIA PaCa-2 cells were treated with BCNU (25 μM), 2DG (25 mM), or siGR and ascorbate (20 mM) for 1 h, and the concentration of intracellular H2O2 was measured. All cells treated with a combination of their respective inhibitor of hydroperoxide metabolism and ascorbate showed significant increases in intracellular H2O2 concentrations when compared to the inhibitors of hydroperoxide removal alone (Means ± SEM, n = 3). F. MIA PaCa-2 cells were treated with pyruvate (5 mM) and ascorbate (20 mM) for 1 h, and the concentration of intracellular H2O2 was measured. Cells treated with a combination of pyruvate and ascorbate showed no change in intracellular H2O2 concentration when compared to control cells (Means ± SEM, n = 3).