Figures & data
Table 1. Effect of AlCl3 concentration of percentage yield, particle size, and drug entrapment efficiency of CMLBG beads loaded with 30% (w/w) glipizide.
Figure 3. Scanning electron micrographs of blank and glipizide-loaded CMLBG bead. Key: Concentration of aluminium chloride: (a) 5% (blank bead); (b) 1%; (c) 3%; (d) 5%.
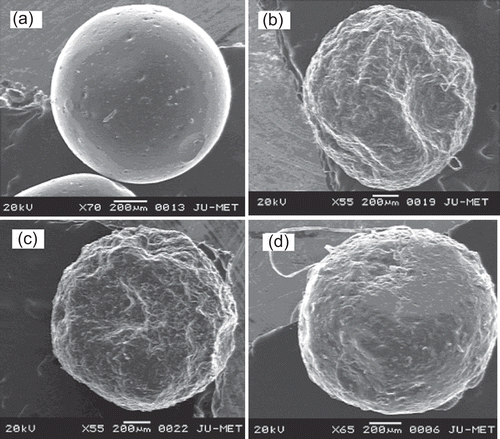
Table 2. Release characteristics and mathematical modeling of the release data of glipizide-loaded CMLBG beads in pH 7.4 phosphate buffer solution.
Figure 4. Release profiles of glipizide-loaded beads in different dissolution media. Drug release in pH 7.4 phosphate buffer solution; Key: Concentration of aluminium chloride: (Δ) 1%; (□) 3%; (○) 5%. Drug release in pH 1.2 KCl-HCl buffer solution; Key: Concentration of aluminium chloride: (▴) 1%; (▪) 3%; (•) 5%.
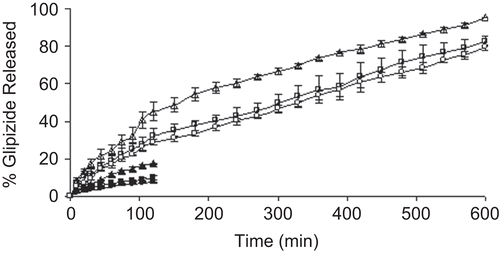
Figure 5. Swelling study of blank beads in different dissolution media. Swelling study in pH 1.2 KCl-HCl buffer solution; Key: Concentration of aluminium chloride: (Δ) 1%; (□) 3%; (○) 5%. Swelling study in pH 7.4 phosphate buffer solution; Key: Concentration of aluminium chloride: (▴) 1%; (▪) 3%; (•) 5%.
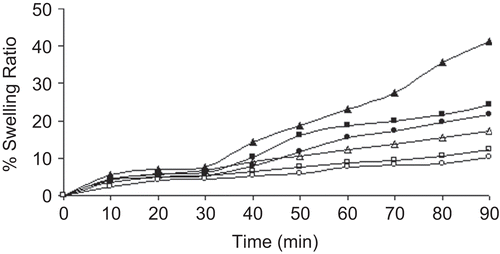
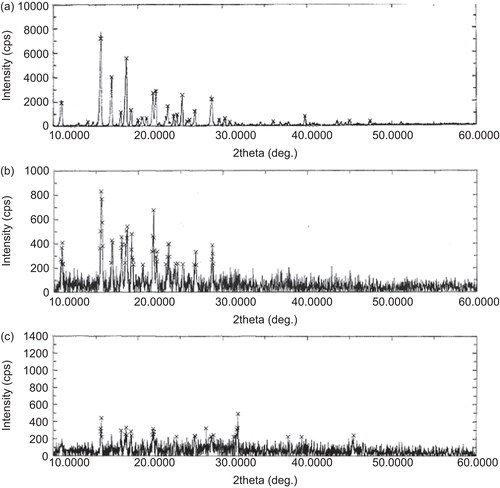
Figure 6. DSC thermograms and TGA curves of blank beads prepared using different concentrations of aluminium chloride. Key: Concentration of aluminium chloride: (a) 1%; (b) 3%; (c) 5%.
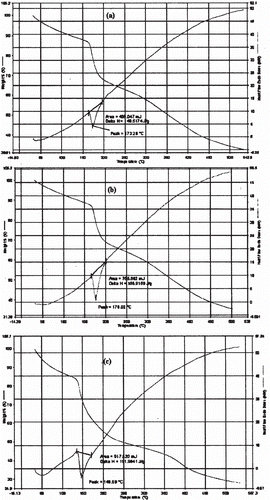
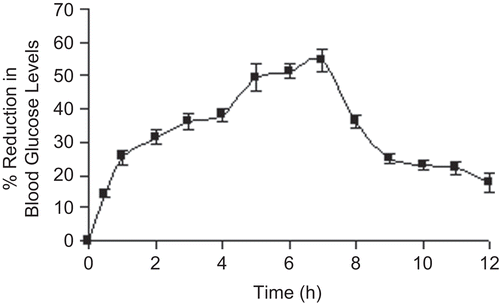
Figure 7. Release profile of glipizide-loaded beads (5% aluminium chloride) in pH 7.4 phosphate buffer solution following dissolution in pH 1.2 KCl/HCl buffer solution for 2 h.
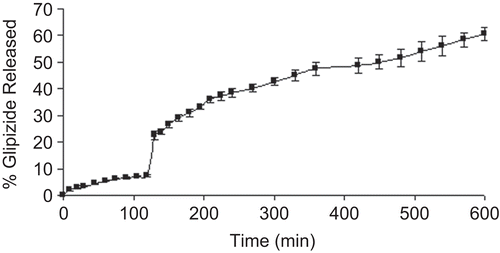
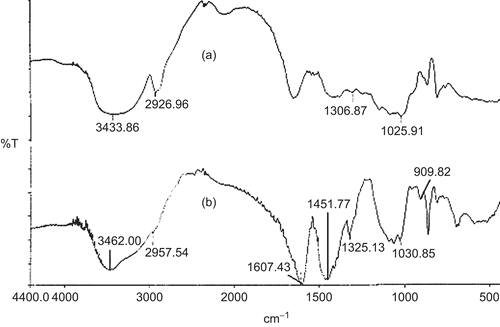
Figure 8. FTIR spectra of (a) blank beads; (b) pure glipizide; (c) glipizide-loaded beads; (d) physical mixture of glipizide and CMLBG.
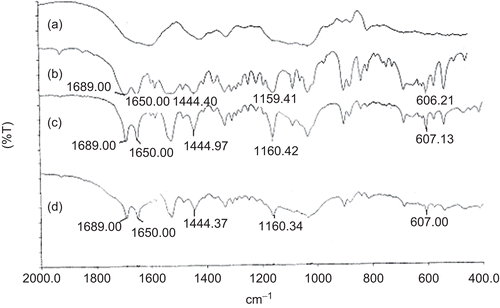
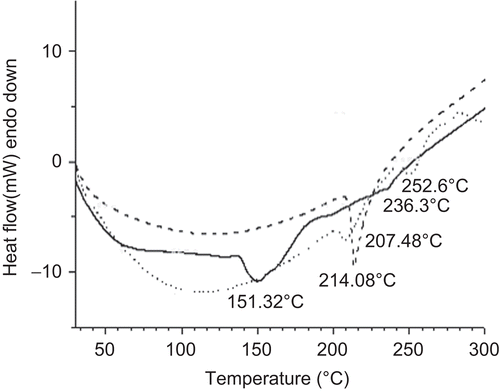
Figure 9. DSC thermograms of pure glipizide (bold dotted line), physical mixture of drug and polymer (faint dotted line), and glipizide-loaded beads (bold continuous line).