Figures & data
Figure 2. (a) Drug particles encapsulated in a nanocapsule, (b) Drug particles dispersed in a nanosphere.
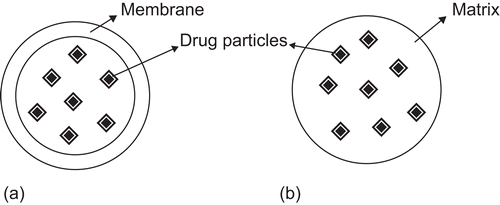
Figure 5. Solid lipid production by liquid flow-focusing and gas displacement method (adopted from CitationYun et al., 2009).
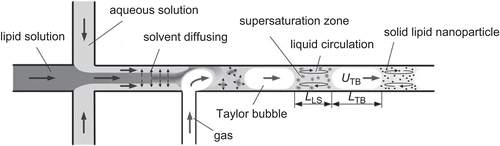
Figure 6. Drug expulsion during storage (modified from CitationMüller et al., 2002).
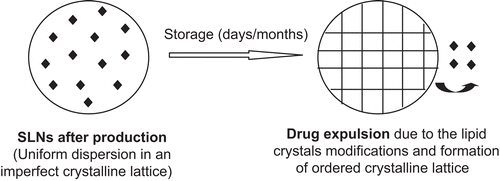
Figure 7. Explanation of burst release associated with solid lipid nanoparticles release profile (adopted from CitationMüller et al., 2002).
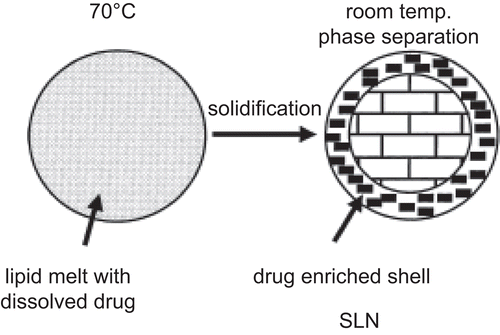
Table 1. Lipids used in the production of SLNs for ocular drug and gene delivery.
Table 2. Emulsifiers used in the production of SLNs for ocular drug delivery.
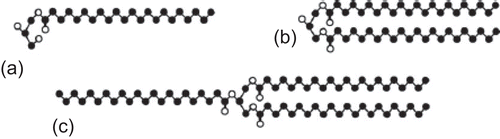
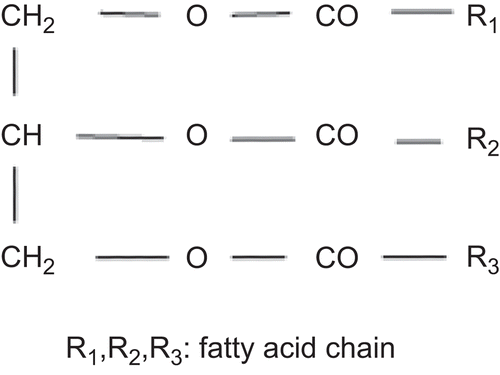
Figure 9. Compritol 888 ATO consists of mixture of monobehenate (a), dibehenate (b), and tribehenate (c) of glycerol (adopted from Brubach et al., 2007).