Figures & data
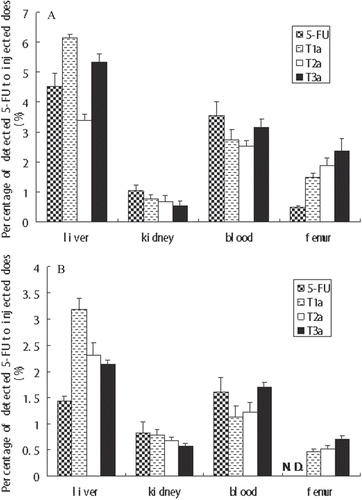
Figure 2. (A) Percentage of conjugates bound to HAP at 1 and 24 h. (B) Change of percent conjugates T1a, T2a, and T3a bound to HAP. Error bars represent the mean and SD of three independent experiments.
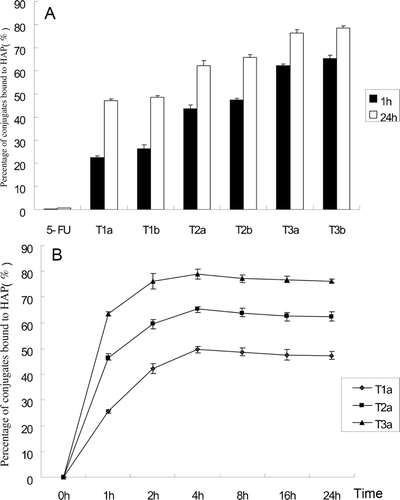
Table 1. Solubility of T1a-b, T2a-b, T3a-b in water (pH=7).
Figure 3. Release of 5-FU from compound T3a (A) and compound T3b (B) in physiological conditions. Error bars represented the mean and standard deviation of three independent experiments. 5-FU, 5-fluorouracil.
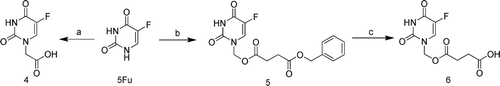
Scheme 1. The synthesis of compound 4–6. Reagents and Conditions: (a) BrCH2COOH, KOH,40°C; (b) i, HCHO, 60°C; ii, PhCH2OOCCH2CH2COOH, CH3CN, DCC, DMAP, r.t.; (c) 10% Pd/ C, CH3OH, r.t. DCC, dicyclohexylcarbodiimide; DMAP, dimethylamino-pyridine.
Scheme 2. The synthesis of compound 3. Reagents and Conditions: (a) i, TFA, CH2Cl2, r.t.; ii, Boc-Asp(OBzl)-OH, IBCF, NMM, THF; (b) i, TFA, CH2Cl2,r.t.; ii, Boc-Gly-OH, IBCF, NMM, THF; (c) i, TFA, CH2Cl2,r.t.; ii, Boc-Arg(NO2)-OH, IBCF, NMM, THF. IBCF, isobutyl chloroformate; NMM, N-methyl-morpholine; TFA, trifluoroacetic acid.
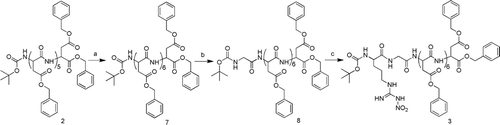
Scheme 3. The synthesis of compounds T1a-b, T2a-b, T3a-b. Reagents and Conditions: (a) i, TFA, CH2Cl2,r.t.; ii, 4, IBCF, NMM, THF; iii, 10% Pd/ C, CH3OH, r.t.; (b) i, TFA, CH2Cl2,r.t.; ii, 6, IBCF, NMM, THF; iii, 10% Pd/ C, CH3OH, r.t. IBCF, isobutyl chloroformate; NMM, N-methyl-morpholine; TFA, trifluoroacetic acid.
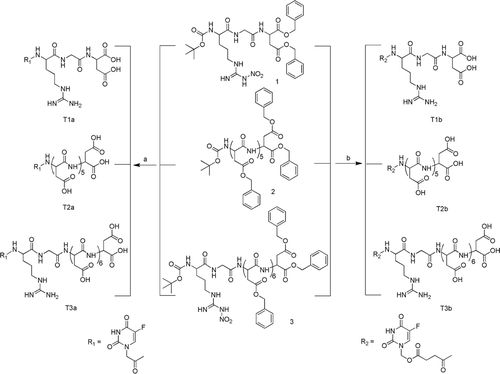
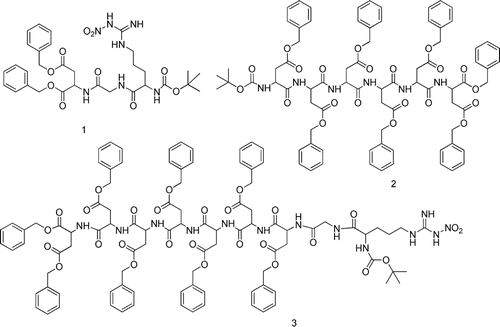
Table 2. Pharmacokinetic parameters of 5-FU and 5-FU-oligopeptides conjugate T1a, T2a, and T3a in rats.