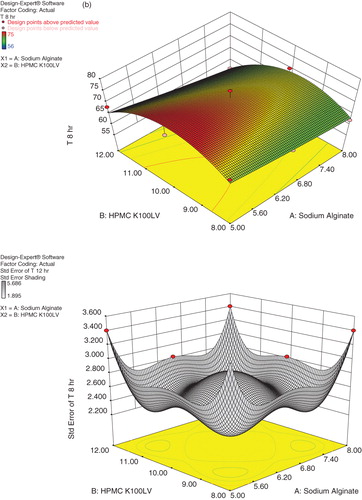
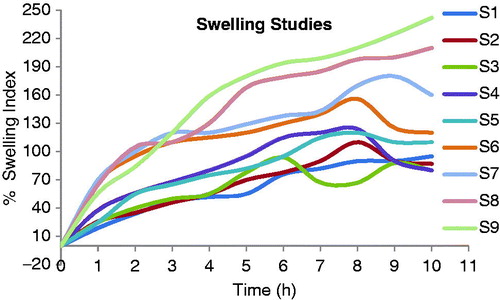
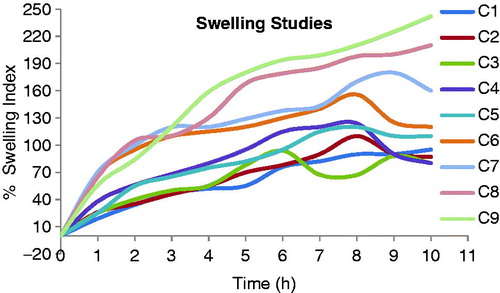
Figures & data
Figure 1. (a) FTIR spectrum of Cefpodoxime Proxetil; (b) FTIR spectrum of drug–excipients mixture; (c) FTIR spectrum of excipients mixture.
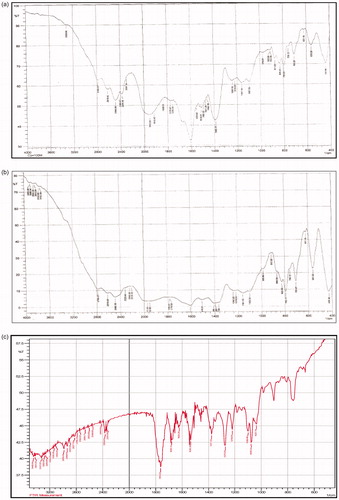
Figure 2. (a) DSC thermogram of Cefpodoxime Proxetil; (b) DSC thermogram of drug–excipients mixture.
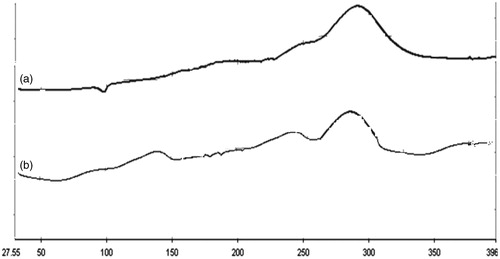
Table 1. Composition of Cefpodoxime Proxetil mucoadhesive tablets (S1–S9).
Table 2. Composition of Cefpodoxime Proxetil mucoadhesive tablets (C1–C9).
Table 3. Evaluation of powder blend of Cefpodoxime Proxetil mucoadhesive tablets (S1–S9).
Table 4. Evaluation of powder blend of Cefpodoxime Proxetil mucoadhesive tablets (C1–C9).
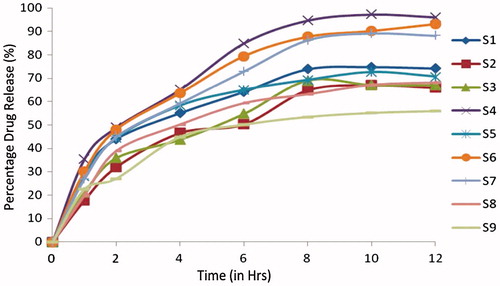
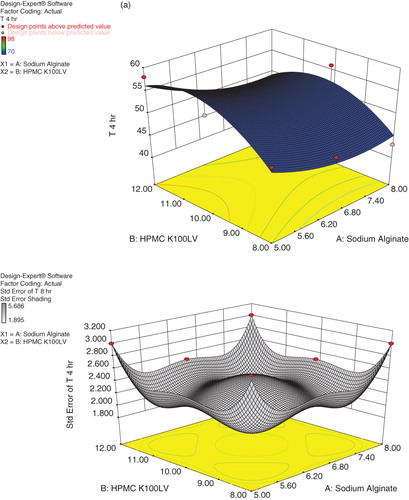
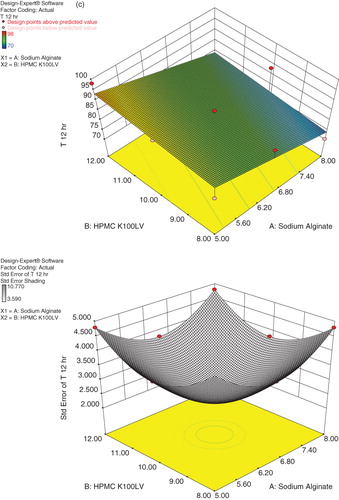
Table 5. Evaluation parameters of Cefpodoxime Proxetil mucoadhesive tablets (S1–S9).
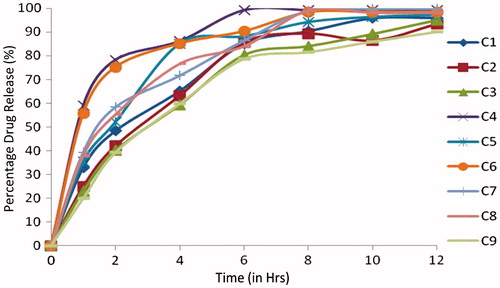
Table 6. Evaluation parameters of Cefpodoxime Proxetil mucoadhesive tablets (C1–C9).
Figure 8. X-ray photographs of Cefpodoxime Proxetil mucoadhesive tablets at different time intervals. (a) at 0 h; (b) after 2 h; (c) after 4 h; (d) after 6 h; (e) after 8 h; (f) after 10 h.
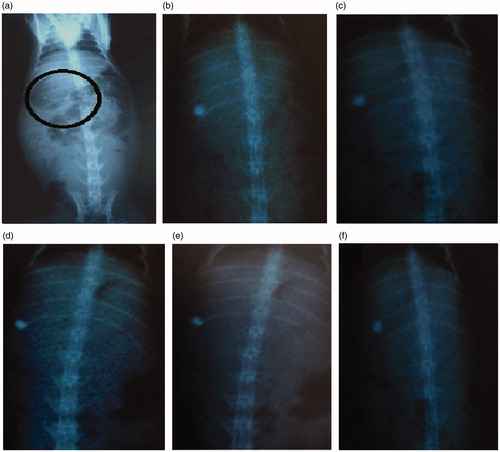
Table 7. Evaluation of Cefpodoxime Proxetil mucoadhesive tablets (S7) kept for stability at 40 °C/75% relative humidity.