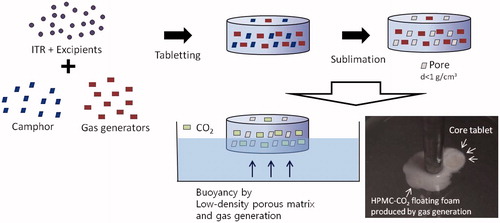
Figures & data
Table 1. Compositions of tablets containing ITR-SD (unit: mg/tablet).
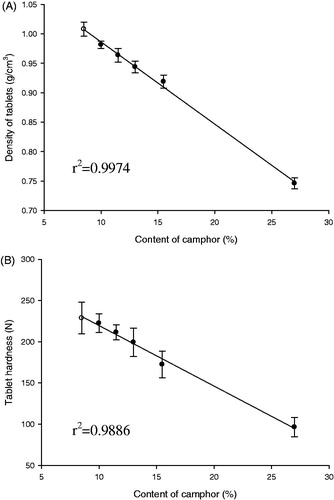
Table 2. Formulations to evaluate effect of camphor amount on hardness and density of LD-GRT (unit: mg/tablet).
Figure 2. DSC thermograms of (A) ITR-SD, (B) physical mixture, (C) HPMC, (D) raw ITR substance and (E) citric acid.
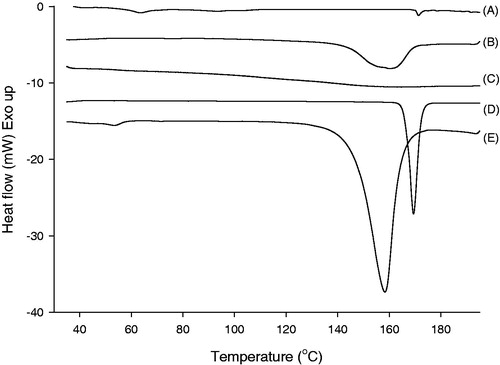
Figure 3. XRD patterns of (A) ITR-SD, (B) physical mixture, (C) HPMC, (D) raw ITR substance and (E) citric acid.
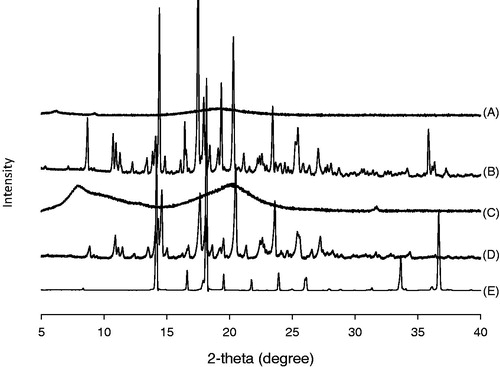
Figure 4. Release profiles of IRT and GG-GRT with various amount of gas-generators (n = 3, Mean ± SD) Key •: F1 (without gas generators), ○: F2 (60 mg of NaHCO3 and 30 mg of citric acid), ▾: F3 (120 mg of NaHCO3 and 60 mg of citric acid), △: IRT.
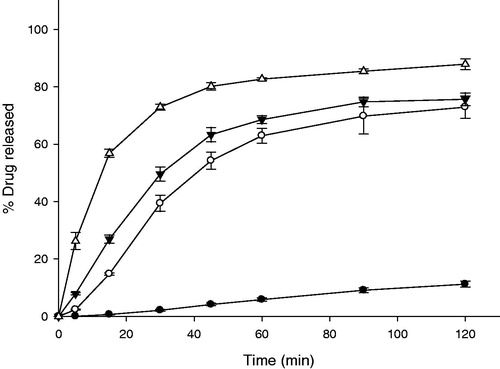
Table 3. Physical properties and release rate of tablets containing ITR-SD.
Figure 5. Release profiles of GG-GRT with various cellulose-derived polymers (n = 3, Mean ± SD) Key •: F3 (HPMC 4,000 cP), ○: F4 (HPMC 15,000 cP), ▾: F5 (HPMC 100,000 cP), △: F6 (HEC).
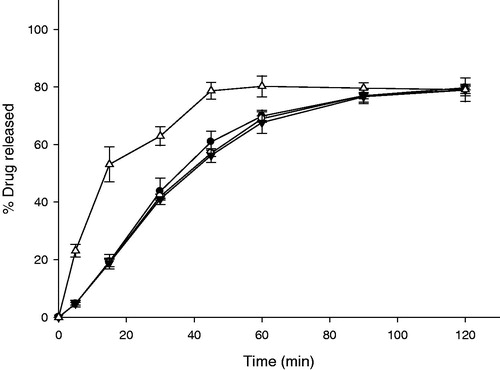
Figure 7. Release profiles of LD-GRT (F8-F10) and DF-GRT with both gas-generators and camphor (F11) (n = 3, Mean ± SD) Key •: F7 (30 mg of HPMC 50 cP without CCS), ○: F8 (30 mg of HPMC 50 cP and 7 mg of CCS), ▾: F9 (30 mg of HPMC 50 cP and 15 mg of CCS), △: F10 (30 mg of HPMC 4000 cP, 15 mg of CCS) and ▪: F11 (DF-GRT).
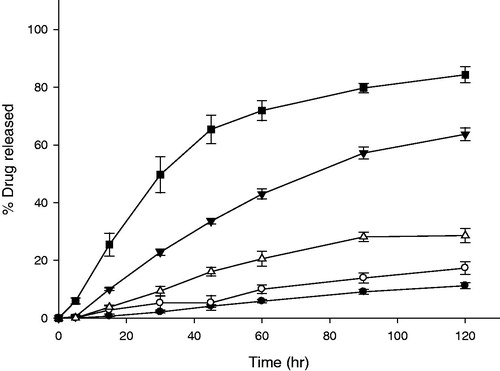
Figure 8. (A) Images of DF-GRT (F11) during in vitro dissolution test. (B) A diagram of GRT (F11) during dissolution test.
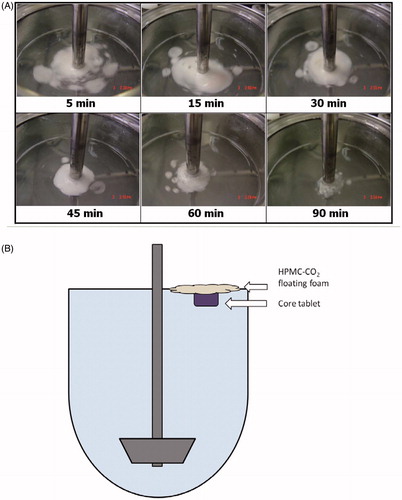
Figure 9. (A) Surface and (B) cross-sectional SEM images of DF-GRT (F11) (magnification: ×25, scale bar: 1 mm).
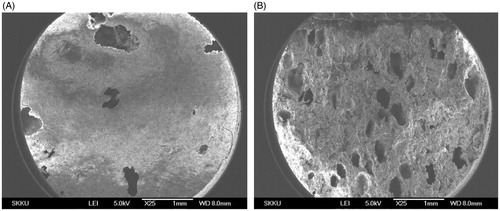
Figure 10. Representative chromatograms of (A) camphor standard solution (1 μg/mL) and (B) sample solution (F11).
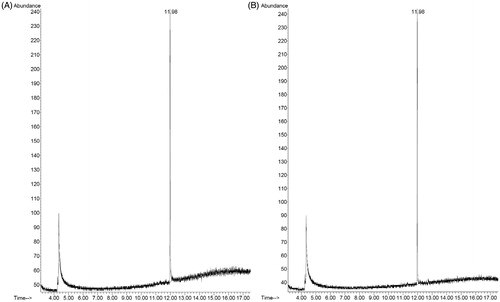
Figure 11. Mean plasma concentration of ITR versus time profiles after oral administration in mini pig model. Key •: IRT, ○: DF-GRT (F11) (n = 6, Mean ± SE).
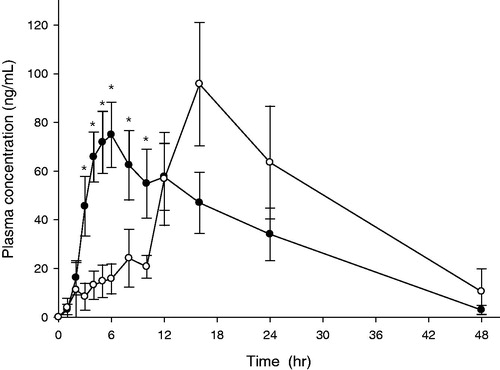
Table 4. Pharmacokinetic parameters for IRT and FD-GRT (F11) containing ITR-SD in the miniature pig model (n = 6, Mean ± SE).