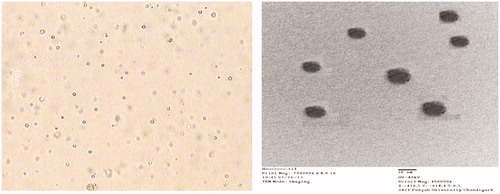
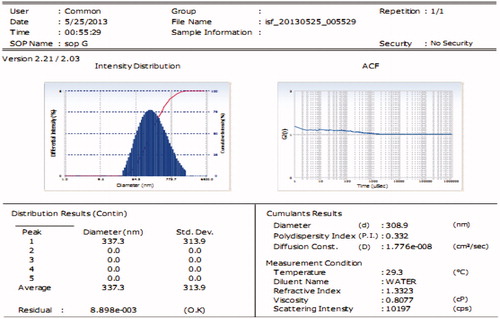
Figures & data
Table 1. Variables in Box–Behnken design.
Table 2. Parameters used for spreadability determination of gel.
Table 3. Different animal groups for in vivo study.
Table 4. Observed responses for BPO-loaded niosomes (Box–Behnken design).
Table 5. Summary of results of regression analysis for responses Y1, for fitting to quadratic model.
Table 6. Summary of results of regression analysis for responses Y2, for fitting to quadratic model.
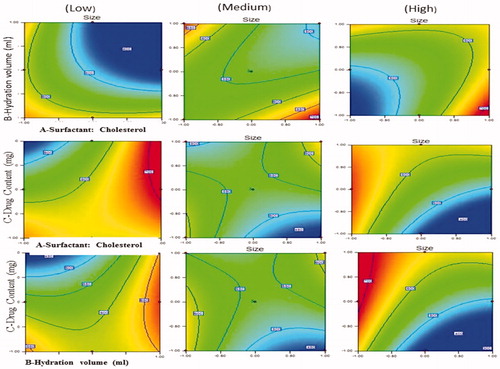
Figure 2. Response surface plot showing effect of interaction between surfactant:cholesterol ratio (A) and hydration volume (B) with low level of drug content (C) on response (size).
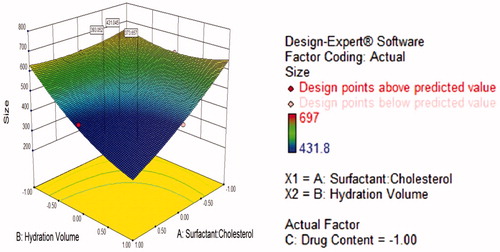
Figure 3. Response surface plot showing effect of surfactant:cholesterol (A) and drug content (C) interaction with high level of hydration volume (B) on response (size).
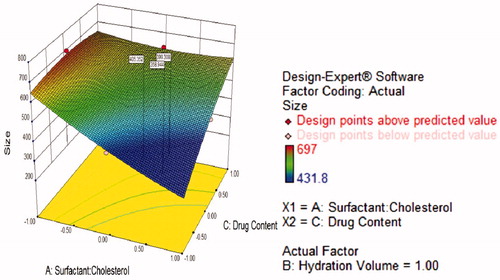
Figure 4. Response surface plot showing effect of hydration volume (B) and drug content (C) interaction with high level surfactant:cholesterol (A) of on response (size).
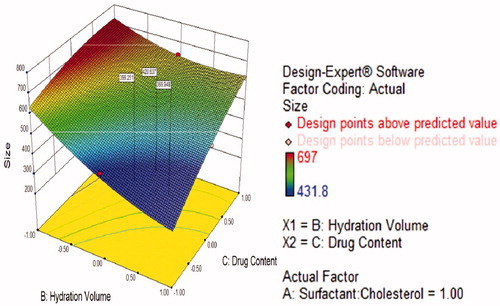
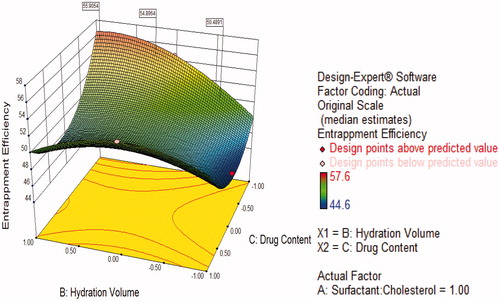
Figure 5. Contour plots showing the interaction effect for entrapment efficiency (orange yellow region shows more entrapment efficiency).
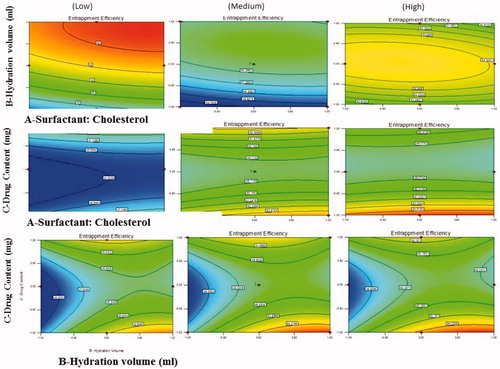
Figure 6. Response surface plot showing effect of interaction between surfactant:cholesterol ratio (A) and hydration volume (B) with low level of drug content (C) on response (entrapment efficiency).
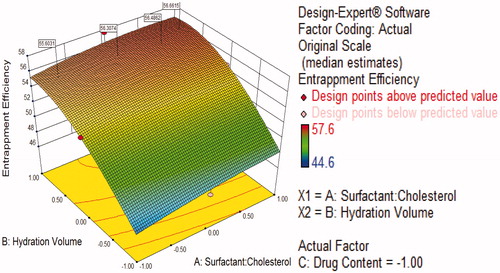
Figure 7. Response surface plot showing effect of interaction between surfactant:cholesterol ratio (A) and drug content (C) with high level of hydration volume (B) on response (entrapment efficiency).
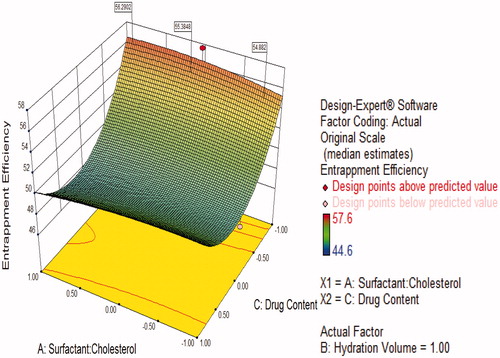
Figure 8. Response surface plot showing effect of interaction between hydration volume (B) and drug content (C) with high level of surfactant:cholesterol ratio (A) on response (entrapment efficiency).