Figures & data
Table 1. Levels and factors in the orthogonal design.
Table 2. The test of orthogonal design for optimization of OP liposome formulation.
Table 3. Results of the orthogonal design of OP liposomes.
Table 4. The entrapment efficiency of liposomes (n = 3).
Table 5. Particle size, polydispersity index and zeta potential of OP liposomes.
Table 6. The physical and chemical properties of liposomal OP dry powders as different ratio of l-leucine and solid content liposome.
Table 7. Properties of optimized liposomal OP dry powders formulation.
Table 8. The solution of liposomes before and after spray-dried at 4 °C.
Table 9. Drug content uniformity of liposomal OP dry powders.
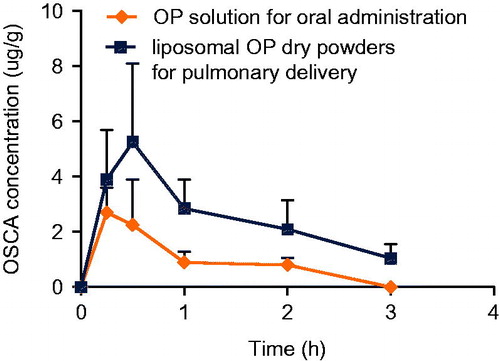
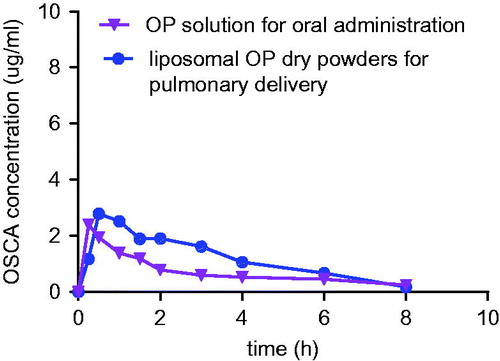
Figure 16. OSCA plasma concentration-time profile of group a (OP solution for oral administration) and group b (liposomal OP dry powders for pulmonary delivery).


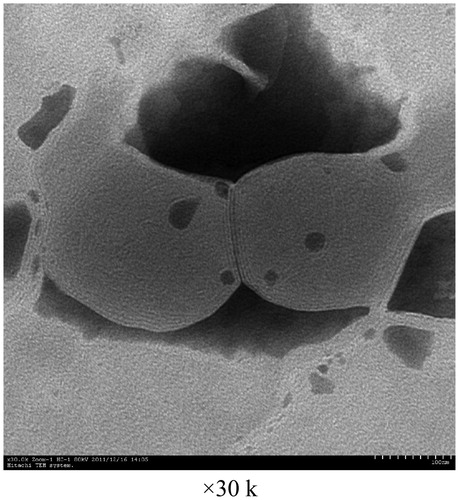
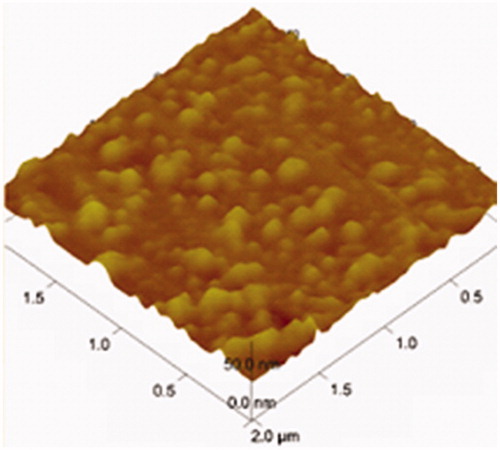
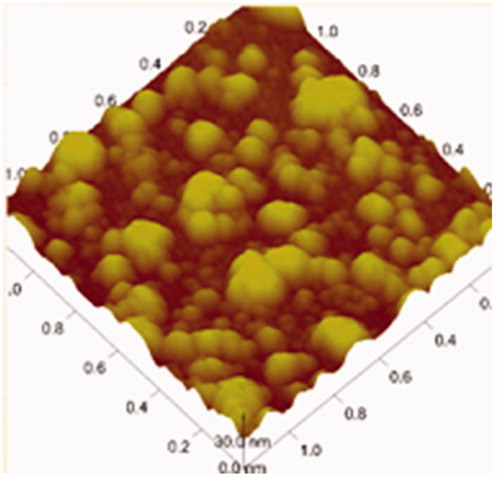
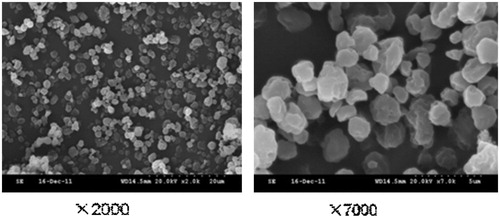
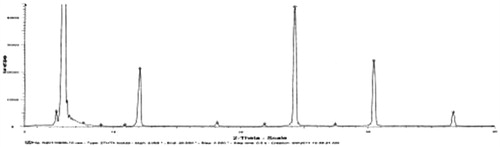
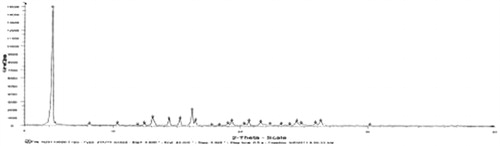

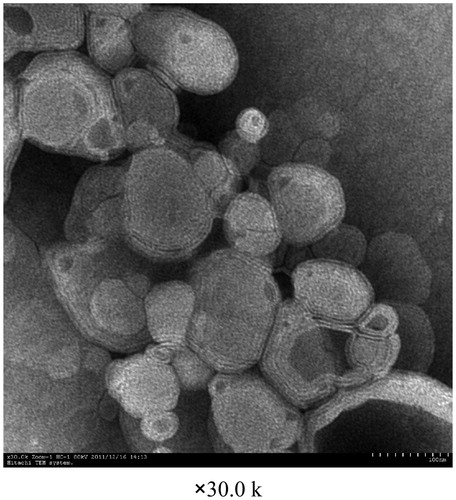
![Figure 15. Regression line of v and v/[s] of liver homogenates and lung homogenates.](/cms/asset/7f74516d-8d6d-4822-8f7b-d20665dc0eb6/idrd_a_863526_f0015_b.jpg)