Figures & data
Table 1. Spray drying process parameter.
Table 2. The magnitude of the impact of each factor by calculation and Yates’s treatment (mean ± SD, n = 3).
Table 3. Formulation parameter and response of a budesonide dry powder inhaler (mean ± SD, n = 3).
Figure 1. Drug–carrier detachment test by ultracentrifugation; (a) two tubes made up of steel were inserted in a plastic centrifuge tube and the double sieves was sandwiched in a middle of the two half cut tube and (b) diagram showing the experimental setup. The centrifuge tube was placed in the rotor of the centrifuge, the position of the powder on the sieves determining the radius of centrifugation (d). During centrifugation, a force (F), proportional to the angular velocity (ω), will pull on the powder with an angle of 180 °C.
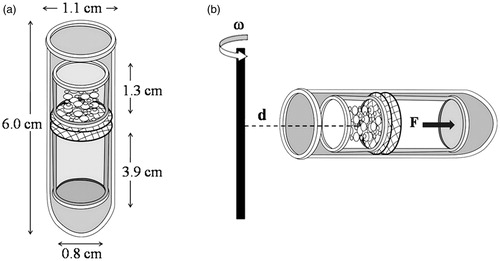
Figure 2. IR spectrum of (a) kneaded budesonide-l-leucine at a ratio of 1:1, (b) spray dried l-leucine and (c) micronized budesonide.
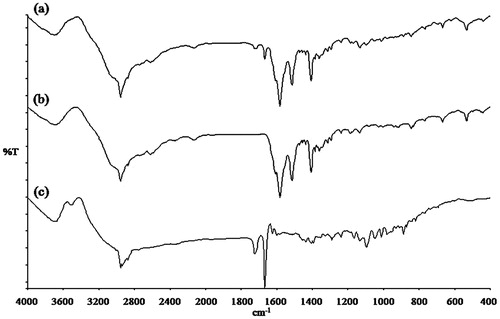
Figure 3. Thermogram of (a) spray dried l-leucine, (b) micronized budesonide, (c) co-spray dried budesonide-l-leucine at a ratio of 1:50 and (d) kneaded budesonide-l-leucine at a ratio of 1:1.
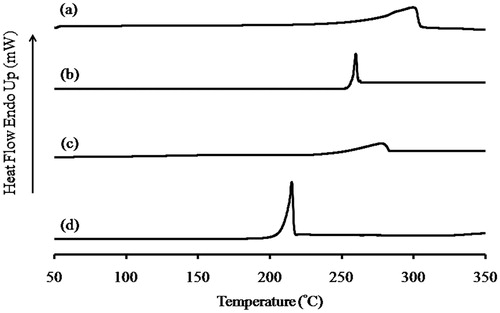
Figure 4. Drug–carrier detachment forces of budesonide dry powder inhalers (Formulations L1, LM1, LM2 and LM3) by ultracentrifugation (mean ± SD, n = 3).
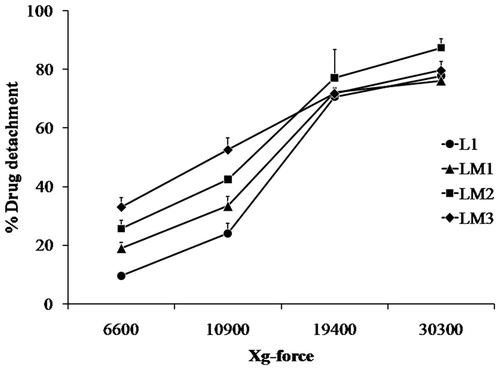
Table 4. Adhesion force of formulations L1, LM1, LM2 and LM3 at 50% of drug detachment (mean ± SD, n = 3).
Table 5. Drug content, coefficient of variation (CV) and in vitro aerosolization properties of the dry powders (mean ± SD, n ≥ 3).
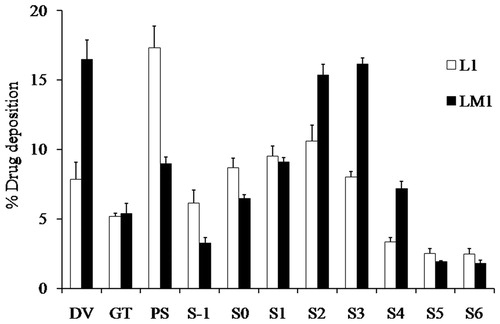
Figure 5. Drug deposition after aerosolization of formulations L1 (white bars) and LM1 (dark bars) into the Andersen cascade impactor at an air flow rate of 60 L/min (mean ± SD, n = 3).