Figures & data
Table 1. Composition of the cefuroxime axetil granules and minitablets.
Table 2. Composition of the cefuroxime axetil minitablets for in vivo bioavailability study.
Table 3. Micromeritic properties of granules.
Table 4. Physical characteristics of minitablets.
Table 5. Parameters related to buoyancy of minitablets.
Figure 1. Photographs of floating of sustained release (SR) minitablets of formulation F3 at 0, 6, and 12 h.
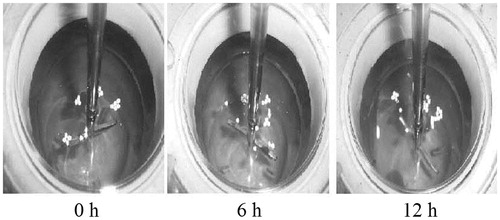
Figure 2. (A) In vitro dissolution profile of Immediate release (IR) formulations. (B) In vitro dissolution profile of sustained release (SR) formulations. (C) In vitro dissolution profile of optimized minitablets (IR and SR) filled in 0 size capsules.
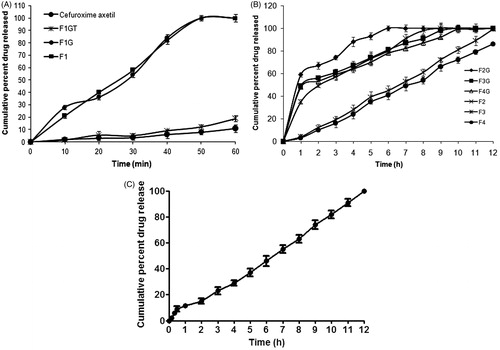
Table 6. In vitro drug release kinetics of formulations.
Figure 3. (A) FT-IR spectra of cefuroxime axetil, gelucire 50/13, and optimized IR minitablet (F1). (B) FT-IR spectra of cefuroxime axetil, gelucire 43/01, and optimized SR minitablet (F3).
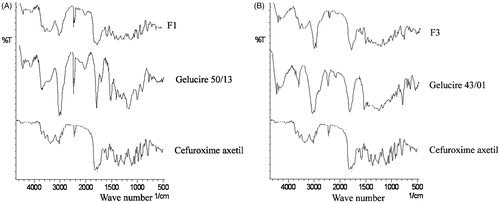
Figure 4. (A) and (B) SEM photographs of granules of optimized IR minitablets (F1) and SR minitablets (F3).

Figure 5. In vitro dissolution profile of optimized formulation F5C at 0 time and after three months of storage at 40 °C/75% RH and ambient conditions.
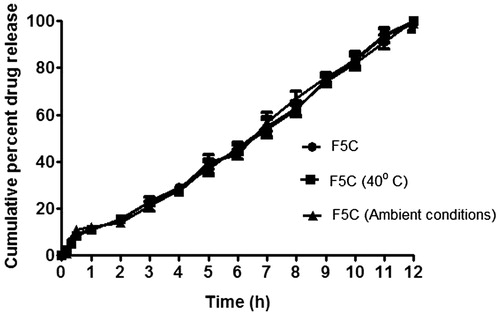
Table 7. Inhibition of growth of in vitro dissolution samples against E. coli.
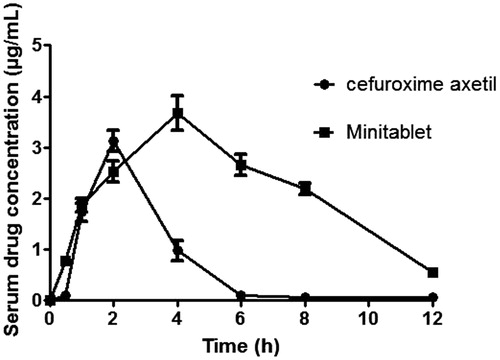
Figure 6. In vivo bioavailability studies for pure drug cefuroxime axetil and optimized minitablet formulation.