Figures & data
Table 1. The composition of the investigated different formulations of famotidine minitablets.
Figure 1. Effect of different kinds of hydrogel matrix material (HPMC K4M and Carbopol 971 P) on swelling behavior showing swelling indices (%) of famotidine minitablets under the conditions of 0.1 M HCl and 37 °C (apparatus II, 50 rpm) (mean ± SD, n = 6).
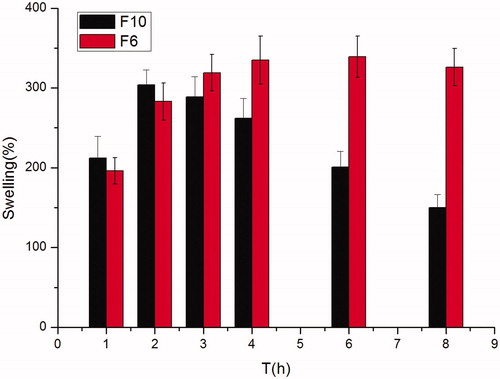
Table 2. The influence of different concentrations of HPMC K4M on the floating lag time and duration of minitablets.
Table 3. The influence of different concentrations of NaHCO3 on the floating lag time and duration of minitablets.
Figure 2. Photographs taken during in vitro buoyancy study of F6 in 150 mL 0.1 M HCl at different time intervals (10 s, 30 s, 60 s, 1 h, 4 h, 8 h).
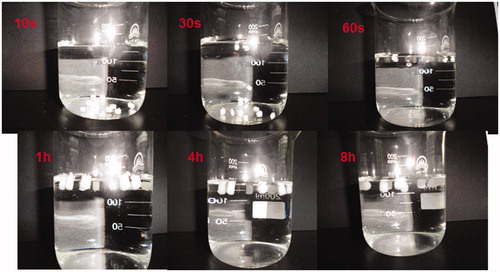
Figure 3. Effect of different concentrations of HPMC K4M (10.00%, w/w; 30.00%, w/w; 50.00%, w/w; 70.00%, w/w) on in vitro release of famotidine minitablets under the conditions of 0.1 M HCl and 37 °C (apparatus II, 50 rpm) (mean ± SD, n = 6).
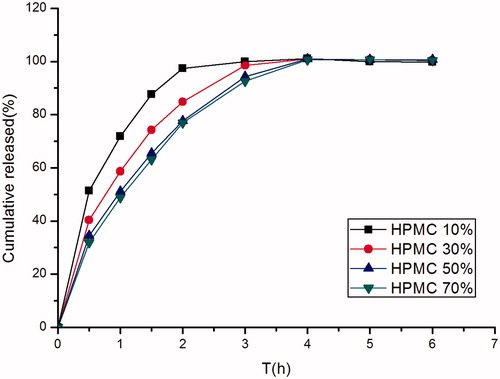
Figure 4. Effect of different kinds of matrix material (HPMC K4M and EC) on in vitro release of famotidine minitablets under the conditions of 0.1 M HCl and 37 °C (apparatus II, 50 rpm) (mean ± SD, n = 6).
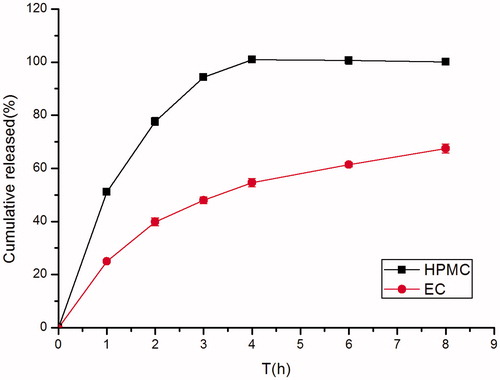
Figure 5. Effect of different concentrations of NaHCO3 (2%, w/w; 5%, w/w; 10%, w/w; 20%, w/w) on in vitro release of famotidine minitablets under the conditions of 0.1 M HCl and 37 °C (apparatus II, 50 rpm) (mean ± SD, n = 6).
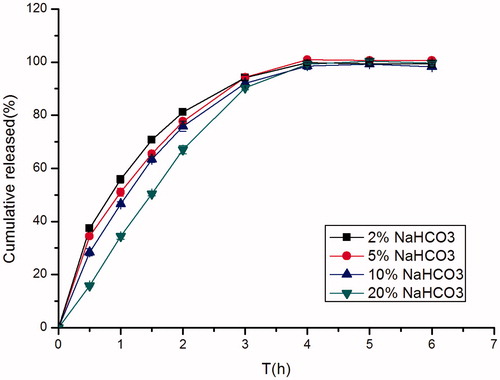
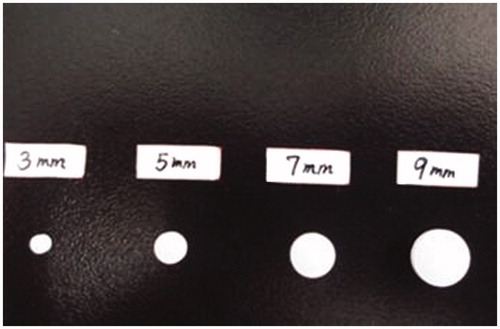
Figure 6. Effect of different diameters (3 mm, 5 mm, 7 mm, 9 mm) on in vitro release of famotidine tablets under the conditions of 0.1 M HCl and 37 °C (apparatus II, 50 rpm) (mean ± SD, n = 6).
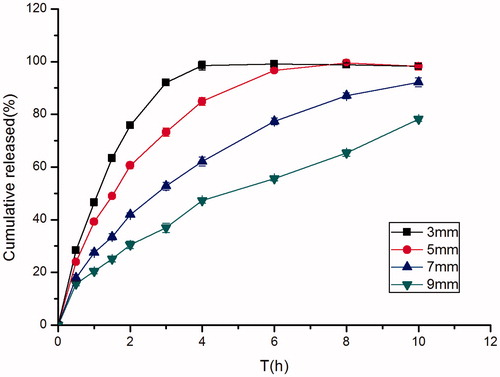
Figure 8. Effect of different dissolution mediums (0.1 M HCl, distilled water, pH 6.8 PBS, pH 7.4 PBS) on in vitro release of famotidine minitablets (apparatus II, 50 rpm) (mean ± SD, n = 6).
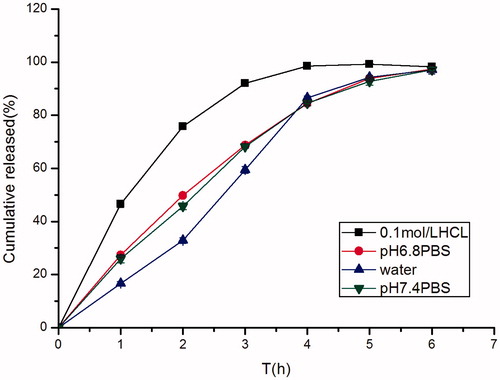
Table 4. R-square of the optimized formulation fitted to zero-order, first-order and Higuchi equations.
Figure 9. Plasma concentration-time profiles of famotidine in rats given famotidine minitablets and reference tablets (XinFaDing®) (mean ± SD, n = 6).
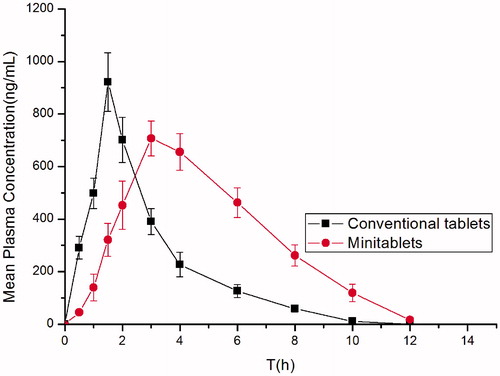
Table 5. The main pharmacokinetic parameters obtained following oral administration of XinFaDing® and the minitablets containing famotidine to rats.