Figures & data
Table 1. Independent factors and observed values of the dependent factors (responses) (n = 3).
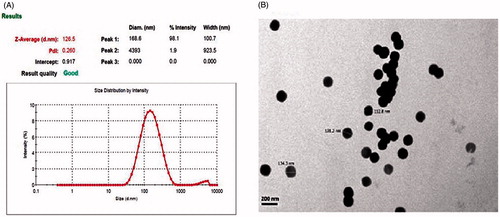
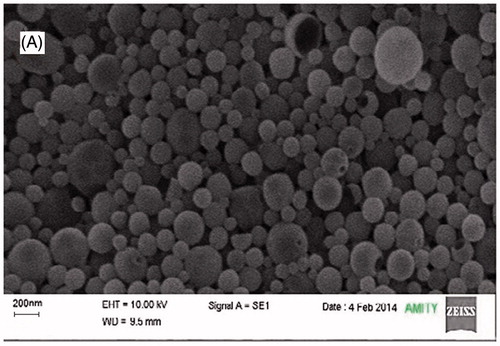
Figure 3. Effects of polymer concentration, stabilizer concentration, and homogenization cycle on particle size.
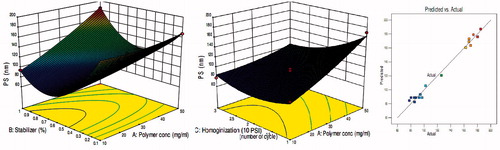
Figure 4. Effects of polymer concentration, stabilizer concentration, and homogenization cycle on entrapment efficiency.
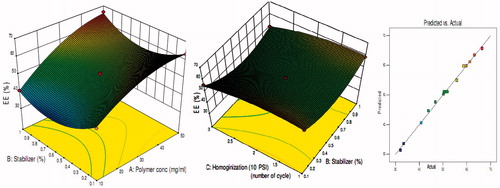
Figure 5. Effects of polymer concentration, stabilizer concentration, and homogenization cycle on loading capacity.
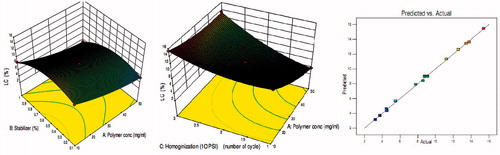
Table 2. Level of independent and dependent variables used in experiments.
Table 3. Final optimization formula for PLGA NPs from point prediction (n = 3).
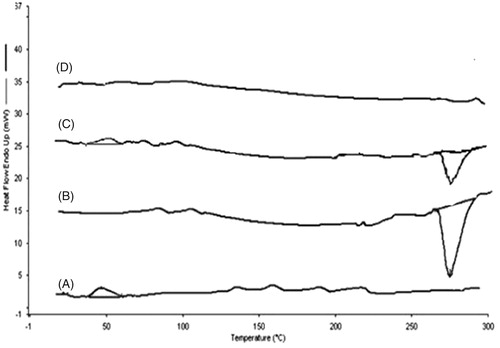
Figure 6. DSC thermograms of PLGA (A), RIS (B), physical mixture of PLGA, and RIS (C), and RIS-loaded PLGA NPs (D).