Figures & data
Table 1. The stability of the (a) Kolliphor HS 15-ARE at room temperature (25 °C) and (b) Kolliphor HS 15-ARE and CA-ARE after dilution.
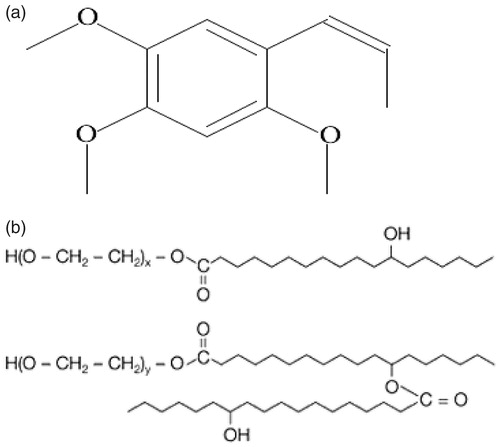
Figure 4. Plot of the fluorescence of pyrene I1/I3 intensity ratio versus concentration of Kolliphor HS 15 in normal saline at 25 °C (n = 3).
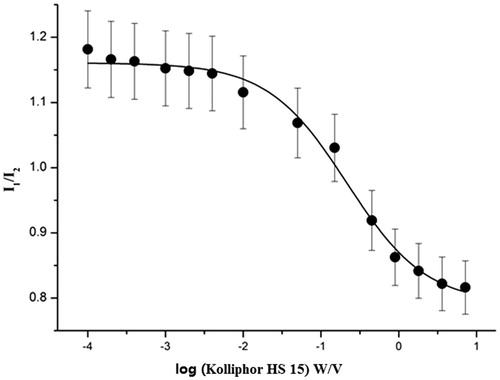
Table 2. Panel a: Design of the hemolysis assay of the Kolliphor HS 15-ARE and Panel b: the hemolysis assay of the Kolliphor HS 15-ARE.
Figure 5. Mean plasma concentration–time curves of ARE in plasma after intravenous injections of HS 15-ARE and CA-ARE. Each value represents the mean±standard deviation (n = 5).
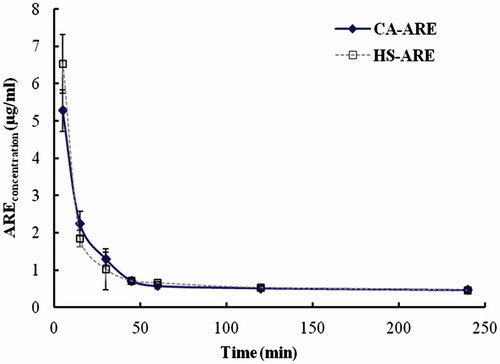
Table 3. The main pharmacokinetic parameters of HS 15-ARE and CA-ARE in rats (n = 5).
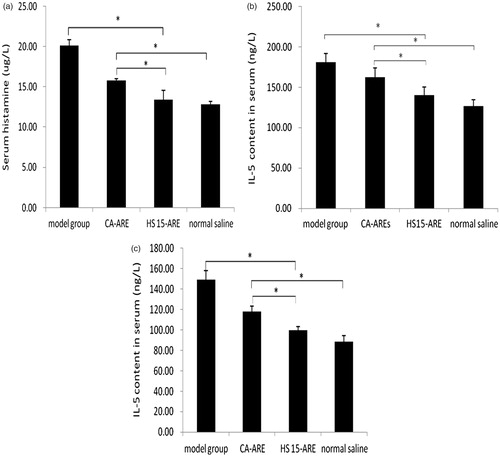
Figure 6. (a) AUC (mg/l min) for HS 15-ARE and CA-ARE in tissue distribution studies (n = 5). *p < 0.05; **p < 0.01; ***p < 0.001 (compared with CA-ARE). (b) The ARE concentration of Kolliphor HS 15-ARE and CA-AREs in tissues at times (10, 30, 60 and 120 min) post-injection (n = 5).
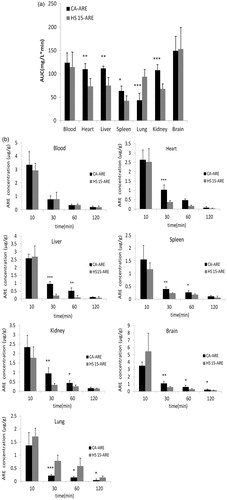
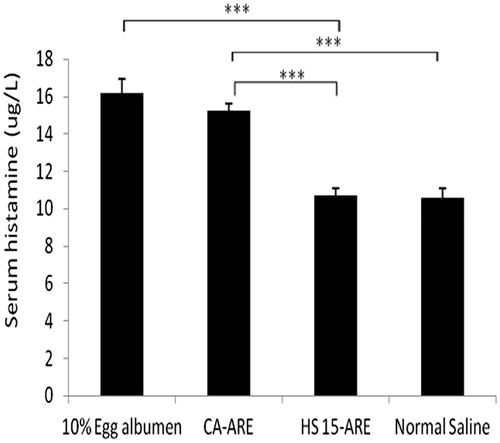
Figure 7. The histamine levels of different groups of guinea pigs in anaphylaxis study. ***p < 0.001 (compared with CA-ARE).