Figures & data
Figure 1. (A) Solution mixture of template, cross-linking monomer, and functional monomers (triangles, squares, circles), (B) complex formation between functional monomers and template via covalent or non-covalent chemistry, (C) the formation of the polymer network typically via free radical polymerization, and (D) template removal step which leaves binding sites specific to the original template (Reproduced with permission from John Wiley & Sons; Kryscio & Peppas, Citation2009).
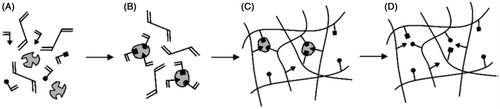
Figure 2. Schematic diagram of four conventional categories of DDS based on mechanism of drug release: diffusion-controlled, swelling-controlled, erosion- controlled and stimuli-controlled systems (Reproduced with permission from John Wiley & Sons; Wang & Von Recum, Citation2011).
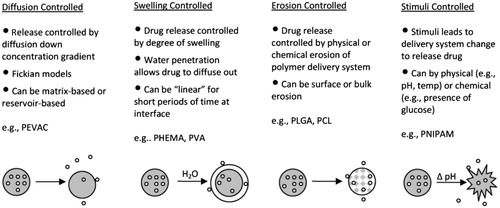
Figure 3. (A) Targeted drug delivery using a molecularly imprinted carrier, (B) targeted drug delivery and facilitated internalization using a MIP (Reproduced with permission from Elsevier Publications; Sellergren & Allender, Citation2005).
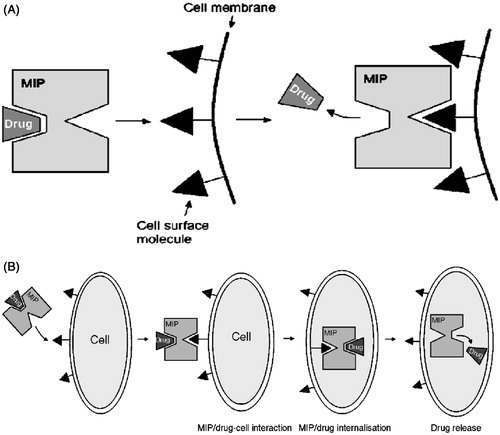
Figure 4. Release characteristics for theophylline-imprinted polymers. Polymers loaded with: Polymer loaded with: ▴, 50 mg theophylline/g polymer; ▪, 10 mg/g; ♦, 2.0 mg/g; ×, 0.1 mg/g (Reproduced with permission from John Wiley & Sons; Norell et al., Citation1998).
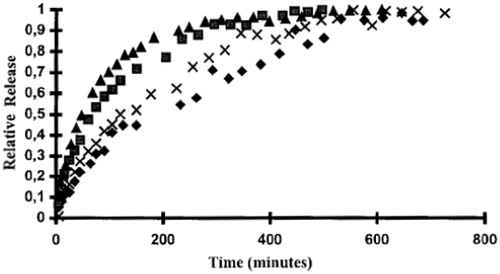
Figure 5. SEM images of membranes: the original bacterial cellulose (A) blank cellulose cast without the addition of PCL-T, (B) blank cellulose cast with the addition of PCL-T, (C) the MIP granule, MIP microsphere and (D–F) surface, (G–I) cross-section, MIP NOM composite cellulose membranes and (J) the enlargement image of MIP-NOM composite membrane (Reproduced with permission from Elsevier Publications; Jantarat et al., Citation2008).
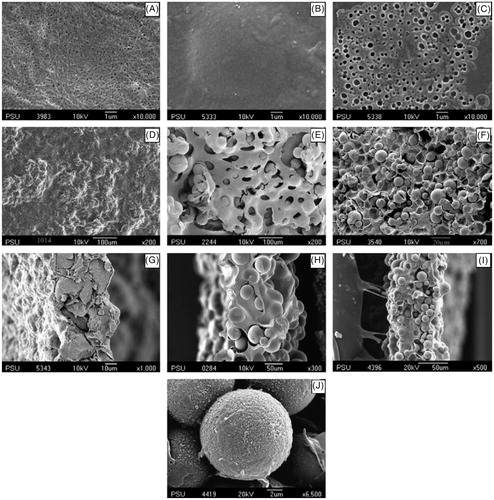
Figure 6. The release profiles of propranolol enantiomers from molecularly imprinted and non-imprinted polymers of (A) granule, (B) microsphere and (C) NOM composite bacterial cellulose membranes at the drug:polymer loading ratios of 1:35 for the granule and 1:100 for microsphere and NOM membranes. The experiment was performed by applying pH 7.4 phosphate buffer as medium to the membranes at room temperature (mean ± S.D., n = 3) (Reproduced with permission from Elsevier Publications; Jantarat et al., Citation2008).
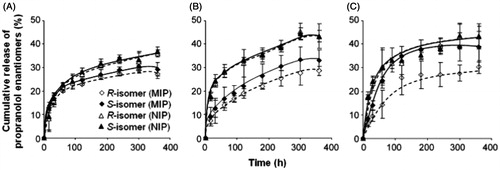
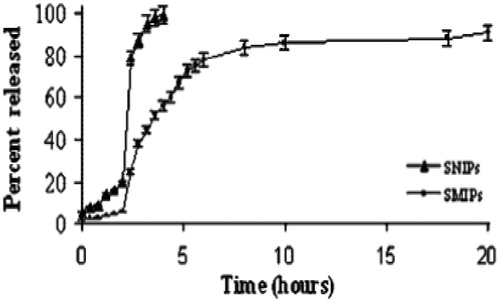
Figure 7. Release profile of sulfasalazine from SMIPs and SNIPs at pH 1 from 0 to 2 h, and at pH 6.8 from 2 to 20 h (Reproduced with permission from John Wiley & Sons; Puoci et al., Citation2004).